Potential Use and Chemical Analysis of Some Natural Plant Extracts for Controlling Listeria spp. Growth In Vitro and in Food
- PMID: 39335846
- PMCID: PMC11431611
- DOI: 10.3390/foods13182915
Potential Use and Chemical Analysis of Some Natural Plant Extracts for Controlling Listeria spp. Growth In Vitro and in Food
Abstract
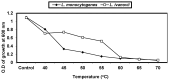
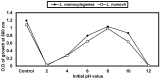
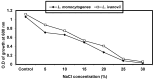
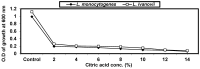
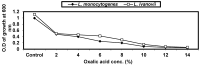
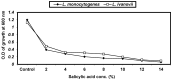
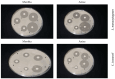
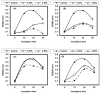
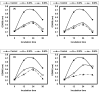
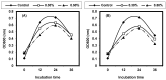
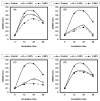
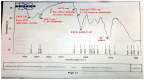
Listeria are Gram-negative intracellular foodborne pathogens that can cause invasive infections with high mortality rates. In this work, the antibacterial activity of ten essential oils, infusion extracts, and decoction extracts of some medicinal plants was tested against Listeria monocytogenes and listeria ivanovii strains. The effects of different physical conditions including temperature, pH, sodium chloride, and some organic acids were studied. The results showed that the water extracts gave the maximum bacterial inhibition, while ethanolic extract was inactive against the tested Listeria spp. The antibiotic sensitivity of L. monocytogenes LMG10470 and L. ivanovii LMZ11352 was tested against five antibiotics including imipenem, levofloxacin, amikacin, ampicillin, and amoxicillin. Imipenem was the most effective antibiotic, resulting in inhibition zones of 40 mm and 31 mm for L. monocytogenes and L. ivanovii, respectively. When imipenem mixed with Syzygium aromaticum oil, Salvia officinalis oil, Pimpinella anisum infusion, and Mentha piperita infusion each, the water extract of Moringa oleifera leaves and seeds against LMG10470 and LMZ11352 resulted in broader antibacterial activity. The antimicrobial activity of both Pimpinella anisum and Mentha piperita plant extracts is related to a variety of bioactive compounds indicated by gas chromatography-mass spectrometry analysis of these two plant extracts. These two plant extracts seemed to contain many chemical compounds elucidated by gas chromatography-mass spectrometry (GC-MS) and infrared radiation spectra. These compounds could be classified into different chemical groups such as ethers, heterocyclic compounds, aromatic aldehydes, condensed heterocyclic compounds, ketones, alicyclic compounds, aromatics, esters, herbicides, saturated fatty acids, and unsaturated fatty acids. The use of these natural compounds seems to be a useful technological adjuvant for the control of Listeria spp. in foods.
Keywords: GC-MS; IR spectra; L. ivanovii LMZ11352; L. monocytogenes LMG10470; essential oils; food-borne bacteria; imipenem; plant extracts.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures










References
-
- Rothrock M.J., Jr., Micciche A.C., Bodie A.R., Ricke S.C. Listeria occurrence and potential control strategies in alternative and conventional poultry processing and retail. Front. Sustain. Food Syst. 2019;3:33. doi: 10.3389/fsufs.2019.00033. - DOI
-
- Bahrami A., Baboli Z.M., Schimmel K., Jafari S.M., Williams L. Efficiency of novel processing technologies for the control of Listeria monocytogenes in food products. Trends Food Sci. Technol. 2020;96:61–78. doi: 10.1016/j.tifs.2019.12.009. - DOI
-
- Dhama K., Karthik K., Tiwari R., Zubair Shabbir M., Barbuddhe S., Singh Malik S.V., Kumar Singh R. Listeriosis in animals, its public health significance (food-borne zoonosis) and advances in diagnosis and control: A comprehensive review. Vet. Q. 2015;35:211–235. doi: 10.1080/01652176.2015.1063023. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

