High-Yield Expressed Human Ferritin Heavy-Chain Nanoparticles in K. marxianus for Functional Food Development
- PMID: 39335848
- PMCID: PMC11431416
- DOI: 10.3390/foods13182919
High-Yield Expressed Human Ferritin Heavy-Chain Nanoparticles in K. marxianus for Functional Food Development
Abstract
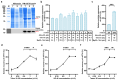
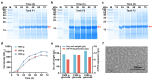
The use of Generally Recognized as Safe (GRAS)-grade microbial cell factories to produce recombinant protein-based nutritional products is a promising trend in developing food and health supplements. In this study, GRAS-grade Kluyveromyces marxianus was employed to express recombinant human heavy-chain ferritin (rhFTH), achieving a yield of 11 g/L in a 5 L fermenter, marking the highest yield reported for ferritin nanoparticle proteins to our knowledge. The rhFTH formed 12 nm spherical nanocages capable of ferroxidase activity, which involves converting Fe2+ to Fe3+ for storage. The rhFTH-containing yeast cell lysates promoted cytokine secretion (tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and -1β (IL-1β)) and enhanced locomotion, pharyngeal pumping frequency, egg-laying capacity, and lifespan under heat and oxidative stress in the RAW264.7 mouse cell line and the C. elegans model, respectively, whereas yeast cell lysate alone had no such effects. These findings suggest that rhFTH boosts immunity, holding promise for developing ferritin-based food and nutritional products and suggesting its adjuvant potential for clinical applications of ferritin-based nanomedicine. The high-yield production of ferritin nanoparticles in K. marxianus offers a valuable source of ferritin for the development of ferritin-based products.
Keywords: Kluyveromyces marxianus; functional foods; nanoparticle; recombinant ferritin.
Conflict of interest statement
Haibo Zhang was employed by the company North America Nutrition Research and Development Society, Guangzhou Aoungo Biotech Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Lucignano R., Stanzione I., Ferraro G., Di Girolamo R., Cané C., Di Somma A., Duilio A., Merlino A., Picone D. A new and efficient procedure to load bioactive molecules within the human heavy-chain ferritin nanocage. Front. Mol. Biosci. 2023;10:1008985. doi: 10.3389/fmolb.2023.1008985. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

