Cardiac Hypertrophy in Pregnant Rats, Descendants of Fructose-Fed Mothers, an Effect That Worsens with Fructose Supplementation
- PMID: 39335874
- PMCID: PMC11431301
- DOI: 10.3390/foods13182944
Cardiac Hypertrophy in Pregnant Rats, Descendants of Fructose-Fed Mothers, an Effect That Worsens with Fructose Supplementation
Abstract
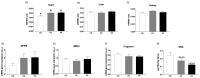
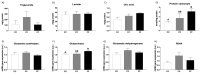
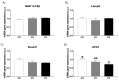
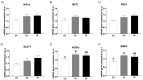
The role of fructose consumption in the development of obesity, MetS, and CVD epidemic has been widely documented. Notably, among other effects, fructose consumption has been demonstrated to induce cardiac hypertrophy. Moreover, fructose intake during pregnancy can cause hypertrophy of the maternal heart. Our previous research has demonstrated that maternal fructose intake has detrimental effects on fetuses, which persist into adulthood and are exacerbated upon re-exposure to fructose. Additionally, we found that maternal fructose consumption produces changes in female progeny that alter their own pregnancy. Despite these findings, fructose intake during pregnancy is not currently discouraged. Given that cardiac hypertrophy is a prognostic marker for heart disease and heart failure, this study aimed to determine whether metabolic changes occurring during pregnancy in the female progeny of fructose-fed mothers could provoke a hypertrophic heart. To test this hypothesis, pregnant rats from fructose-fed mothers, with (FF) and without (FC) fructose supplementation, were studied and compared to pregnant control rats (CC). Maternal hearts were analyzed. Although both FF and FC mothers exhibited heart hypertrophy compared to CC rats, cardiac DNA content was more diminished in the hearts of FF dams than in those of FC rats, suggesting a lower number of heart cells. Accordingly, changes associated with cardiac hypertrophy, such as HIF1α activation and hyperosmolality, were observed in both the FC and FF dams. However, FF dams also exhibited higher oxidative stress, lower autophagy, and decreased glutamine protection against hypertrophy than CC dams. In conclusion, maternal fructose intake induces changes in female progeny that alter their own pregnancy, leading to cardiac hypertrophy, which is further exacerbated by subsequent fructose intake.
Keywords: cardiac hypertrophy; fetal programming; fructose; pregnancy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Pregnancy Is Enough to Provoke Deleterious Effects in Descendants of Fructose-Fed Mothers and Their Fetuses.Nutrients. 2021 Oct 19;13(10):3667. doi: 10.3390/nu13103667. Nutrients. 2021. PMID: 34684668 Free PMC article.
-
Fructose Consumption Affects Placental Production of H2S: Impact on Preeclampsia-Related Parameters.Nutrients. 2024 Jan 20;16(2):309. doi: 10.3390/nu16020309. Nutrients. 2024. PMID: 38276547 Free PMC article.
-
Maternal fructose intake aggravates the harmful effects of a Western diet in rat male descendants impacting their cholesterol metabolism.Food Funct. 2024 Jun 4;15(11):6147-6163. doi: 10.1039/d4fo01466a. Food Funct. 2024. PMID: 38767501
-
Maternal fructose induces gender-dependent changes in both LXRα promoter methylation and cholesterol metabolism in progeny.J Nutr Biochem. 2018 Nov;61:163-172. doi: 10.1016/j.jnutbio.2018.08.011. Epub 2018 Sep 1. J Nutr Biochem. 2018. PMID: 30236873
-
Maternal Fructose Intake Increases Liver H2 S Synthesis but Exarcebates its Fructose-Induced Decrease in Female Progeny.Mol Nutr Food Res. 2020 Sep;64(18):e2000628. doi: 10.1002/mnfr.202000628. Epub 2020 Aug 19. Mol Nutr Food Res. 2020. PMID: 32754997
References
Grants and funding
LinkOut - more resources
Full Text Sources

