Boosting Fructosyl Transferase's Thermostability and Catalytic Performance for Highly Efficient Fructooligosaccharides (FOS) Production
- PMID: 39335925
- PMCID: PMC11431173
- DOI: 10.3390/foods13182997
Boosting Fructosyl Transferase's Thermostability and Catalytic Performance for Highly Efficient Fructooligosaccharides (FOS) Production
Abstract
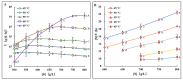
Achieving enzymatic food processing at high substrate concentrations can significantly enhance production efficiency; however, related studies are notably insufficient. This study focused on the enzymatic synthesis of fructooligosaccharides (FOS) at high temperature and high substrate concentration. Results revealed that increased viscosity and limited substrate solubility in high-concentration systems could be alleviated by raising the reaction temperature, provided it aligned with the enzyme's thermostability. Further analysis of enzyme thermostability in real sucrose solutions demonstrates that the enzyme's thermostability was remarkedly improved at higher sucrose concentrations, evidenced by a 10.3 °C increase in melting temperature (Tm) in an 800 g/L sucrose solution. Building upon these findings, we developed a novel method for enzymatic FOS synthesis at elevated temperatures and high sucrose concentrations. Compared to existing commercial methods, the initial transglycosylation rate and volumetric productivity for FOS synthesis increased by 155.9% and 113.5%, respectively, at 65 °C in an 800 g/L sucrose solution. This study underscores the pivotal role of substrate concentration, incubation temperature, and the enzyme's actual status in advancing enzyme-catalyzed processes and demonstrates the potential of enzymatic applications in enhancing food processing technologies, providing innovative strategies for the food industry.
Keywords: engineering process optimization; enzymatic catalysis efficiency; enzyme-catalyzed FOS synthesis; high substrate concentration; thermostability of enzymes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Grants and funding
- JC20200309/the Tianjin Outstanding Talent Program, China
- 2021YFE0106200/the Intergovernmental International Scientific and Technological Innovation Cooperation Program, MOST, China
- 2018YFE0100400/the Intergovernmental International Scientific and Technological Innovation Cooperation Program, MOST, China
LinkOut - more resources
Full Text Sources

