Unveiling the Nutritional Profile and Safety of Coffee Pulp as a First Step in Its Valorization Strategy
- PMID: 39335934
- PMCID: PMC11431805
- DOI: 10.3390/foods13183006
Unveiling the Nutritional Profile and Safety of Coffee Pulp as a First Step in Its Valorization Strategy
Abstract
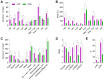
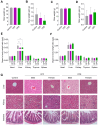
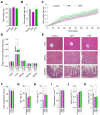
The coffee pulp, a significant by-product of coffee processing, is often discarded but has potential for recycling and high-value uses. This study aimed to investigate the chemical composition of two coffee pulp ingredients, a flour (CPF) and an aqueous extract (CPE), and conducted acute and sub-chronic toxicity assays to determine their safety. The proximate composition revealed the high fiber content of both ingredients; the CPF mainly contained insoluble fiber, while CPE consisted exclusively of soluble pectic polysaccharides. The CPF had higher concentrations of amino acids and a better balance of essential/non-essential amino acids, whereas the CPE exhibited higher concentrations of free amino acids, ensuring higher bioavailability. Both ingredients showed elevated mineral content, while heavy-metal concentrations remained within acceptable limits. This study established the bioactive potential of the CPF and the CPE, demonstrating the high content of caffeine and gallic, protocatechuic, and 4-caffeoylquinic acids. The toxicity studies revealed that the CPF and the CPE exhibited safety when orally administered to mice. Administered doses were non-toxic, as they did not induce lethality or adverse effects in the mice or produce significant histopathological or biochemical adverse changes. This study represents a first step in valorizing the CPF and the CPE as safe novel food ingredients with health benefits for functional and nutritional foods.
Keywords: (poly)phenols; amino acids; bioactive compounds; caffeine; coffee pulp; dietary fiber; food ingredient; minerals; safety; toxicity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ico T., Indicator C., Milds T.C.M., York N., Futures L., York T.N., Milds O., Naturals B., Milds C., America S., et al. Colombian Milds-Other Milds Differential Tightens, I-CIP Averages. 2023. [(accessed on 19 July 2024)]. pp. 1–11. Available online: https://icocoffee.org/documents/cy2022-23/cmr-0323-e.pdf.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

