Theory and Measurement of Heat Transport in Solids: How Rigidity and Spectral Properties Govern Behavior
- PMID: 39336210
- PMCID: PMC11433001
- DOI: 10.3390/ma17184469
Theory and Measurement of Heat Transport in Solids: How Rigidity and Spectral Properties Govern Behavior
Abstract
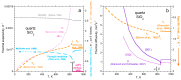
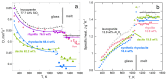
Models of heat transport in solids, being based on idealized elastic collisions of gas molecules, are flawed because heat and mass diffuse independently in solids but together in gas. To better understand heat transfer, an analytical, theoretical approach is combined with data from laser flash analysis, which is the most accurate method available. Dimensional analysis of Fourier's heat equation shows that thermal diffusivity (D) depends on length-scale, which has been confirmed experimentally for metallic, semiconducting, and electrically insulating solids. A radiative diffusion model reproduces measured thermal conductivity (K = DρcP = D × density × specific heat) for thick solids from ~0 to >1200 K using idealized spectra represented by 2-4 parameters. Heat diffusion at laboratory temperatures (conduction) proceeds by absorption and re-emission of infrared light, which explains why heat flows into, through, and out of a material. Because heat added to matter performs work, thermal expansivity is proportional to ρcP/Young's modulus (i.e., rigidity or strength), which is confirmed experimentally over wide temperature ranges. Greater uptake of applied heat (e.g., cP generally increasing with T or at certain phase transitions) reduces the amount of heat that can flow through the solid, but because K = DρcP, the rate (D) must decrease to compensate. Laser flash analysis data confirm this proposal. Transport properties thus depend on heat uptake, which is controlled by the interaction of light with the material under the conditions of interest. This new finding supports a radiative diffusion mechanism for heat transport and explains behavior from ~0 K to above melting.
Keywords: Young’s modulus; absolute methods; diffusion; dimensional analysis; heat; length-scale physics; mechanisms; pressure–volume work; radiative transfer; thermal expansivity.
Conflict of interest statement
The author declares no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures












References
-
- Fourier J.B.J. In: Théorie Analytique de la Chaleur. Chez Firmin Didot, Paris. Freeman A., translator. Dover Publications Inc.; New York, NY, USA: 1822. Translated in 1955 as The Analytic Theory of Heat.
-
- Hust J.G., Lankford A.B. Update of thermal conductivity and electrical resistivity of electrolytic iron, tungsten, and stainless steel. Natl. Bur. Stand. Spec. Pub. 1984;260:90.
-
- Gale W.F., Totemeier T.C. Smithells Metals Reference Book. 8th ed. Elsevier; Amsterdam, The Netherlands: 2004.
-
- Criss E.M., Hofmeister A.M. Isolating lattice from electronic contributions in thermal transport measurements of metals and alloys and a new model. Int. J. Mod. Phys. B. 2017;31:1750205. doi: 10.1142/S0217979217502058. - DOI
-
- Truesdell C. The Tragicomical History of Thermodynamics. Springer; New York, NY, USA: 1980.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

