Valorization of Iron (II) Oxalate Dihydrate Coming from Pickling Processes through Thermal Conversion
- PMID: 39336371
- PMCID: PMC11433084
- DOI: 10.3390/ma17184630
Valorization of Iron (II) Oxalate Dihydrate Coming from Pickling Processes through Thermal Conversion
Abstract
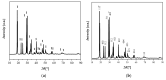
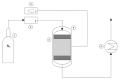
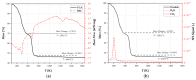
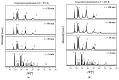
The valorization of industrial byproducts is an emerging practice that aims to transform waste materials generated during production processes into valuable resources. In this work, a preliminary study was carried out on the thermal conversion of an industrial solid byproduct resulting from the pickling of metal surfaces, mainly containing iron (II) oxalate. In a fixed-bed reactor, the thermal conversion was investigated as a function of the operating temperature and overall time. The starting material and the products obtained after heat treatment were characterized in detail, using numerous qualitative and semi-quantitative techniques. The aim of this research was to determine the optimal operating conditions for the transformation of the industrial byproduct into a high-quality product. By varying the operating conditions, it was found that complete conversion of iron (II) oxalate to magnetite was achieved at high temperatures (i.e., 773 K and 873 K) after one hour of treatment. The resulting product had a low degree of crystallization, which increased slightly with an increasing reaction time at a temperature of 873 K, reaching a maximum of about 11%. The magnetite obtained can be used in the future as a starting material for chemical looping processes as a chemical/energy carrier for the production of hydrogen.
Keywords: Iron (II) oxalate; circular economy; thermal treatment.
Conflict of interest statement
The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Zaferani H.S., Sharifi M., Zaarei D., Shishesaz M.R. Application of eco-friendly products as corrosion inhibitors for metals in acid pickling processes—A review. J. Env. Chem. Eng. 2013;1:652–657. doi: 10.1016/j.jece.2013.09.019. - DOI
-
- Sefaja J., Dernikovic B., Malina J., Locrecek B. Investigation of steel corrosion in pickling solutions: Solutions with inhibitors. Surf. Technol. 1983;20:247–263. doi: 10.1016/0376-4583(83)90008-0. - DOI
-
- Liu Y., Wang L., Liu L., Han W., Sun X., Li J., Shen J. Hydrochloric acid pickling process optimization in metal wire working. Int. J. Simul. Syst. Sci. Technol. 2015;16:1.1–1.6. doi: 10.5013/IJSSST.a.16.05.01. - DOI
-
- Yang J.B., Cai N.S., Li Z.S. Hydrogen production from the steam-iron process with direct reduction of iron oxide by chemical looping combustion of coal char. Energy Fuels. 2008;22:2570–2579. doi: 10.1021/ef800014r. - DOI
LinkOut - more resources
Full Text Sources

