GSM1 Requires Hap4 for Expression and Plays a Role in Gluconeogenesis and Utilization of Nonfermentable Carbon Sources
- PMID: 39336719
- PMCID: PMC11432098
- DOI: 10.3390/genes15091128
GSM1 Requires Hap4 for Expression and Plays a Role in Gluconeogenesis and Utilization of Nonfermentable Carbon Sources
Abstract
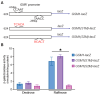
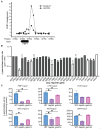
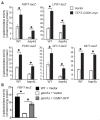
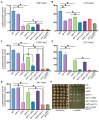
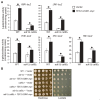
Multiple transcription factors in the budding yeast Saccharomyces cerevisiae are required for the switch from fermentative growth to respiratory growth. The Hap2/3/4/5 complex is a transcriptional activator that binds to CCAAT sequence elements in the promoters of many genes involved in the tricarboxylic acid cycle and oxidative phosphorylation and activates gene expression. Adr1 and Cat8 are required to activate the expression of genes involved in the glyoxylate cycle, gluconeogenesis, and utilization of nonfermentable carbon sources. Here, we characterize the regulation and function of the zinc cluster transcription factor Gsm1 using Western blotting and lacZ reporter-gene analysis. GSM1 is subject to glucose repression, and it requires a CCAAT sequence element for Hap2/3/4/5-dependent expression under glucose-derepression conditions. Genome-wide CHIP analyses revealed many potential targets. We analyzed 29 of them and found that FBP1, LPX1, PCK1, SFC1, and YAT1 require both Gsm1 and Hap4 for optimal expression. FBP1, PCK1, SFC1, and YAT1 play important roles in gluconeogenesis and utilization of two-carbon compounds, and they are known to be regulated by Cat8. GSM1 overexpression in cat8Δ mutant cells increases the expression of these target genes and suppresses growth defects in cat8Δ mutants on lactate medium. We propose that Gsm1 and Cat8 have shared functions in gluconeogenesis and utilization of nonfermentable carbon sources and that Cat8 is the primary regulator.
Keywords: Cat8; Fbp1; Gsm1; Hap2/3/4/5; Hap4; Pck1; S. cerevisiae; gluconeogenesis; transcriptional regulation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Rahner A., Scholer A., Martens E., Gollwitzer B., Schuller H.J. Dual Influence of the Yeast Cat1p (Snf1p) Protein Kinase on Carbon Source-Dependent Transcriptional Activation of Gluconeogenic Genes by the Regulatory Gene Cat8. Nucleic Acids Res. 1996;24:2331–2337. doi: 10.1093/nar/24.12.2331. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

