Limb Perfusion Delivery of a rAAV1 Alpha-1 Antitrypsin Vector in Non-Human Primates Is Safe but Insufficient for Therapy
- PMID: 39336779
- PMCID: PMC11431094
- DOI: 10.3390/genes15091188
Limb Perfusion Delivery of a rAAV1 Alpha-1 Antitrypsin Vector in Non-Human Primates Is Safe but Insufficient for Therapy
Abstract
Background/objectives: α-1 antitrypsin (AAT) deficiency is an inherited, genetic condition characterized by reduced serum levels of AAT and increased risk of developing emphysema and liver disease. AAT is normally synthesized primarily in the liver, but muscle-targeting with a recombinant adeno-associated virus (rAAV) vector for α-1 antitrypsin (AAT) gene therapy has been used to minimize liver exposure to the virus and hepatotoxicity. Clinical trials of direct intramuscular (IM) administration of rAAV1-hAAT have demonstrated its overall safety and transgene expression for 5 years. However, the failure to reach the therapeutic target level after 100 large-volume (1.5 mL) IM injections of maximally concentrated vector led us to pursue a muscle-targeting approach using isolated limb perfusion. This targets the rAAV to a greater muscle mass and allows for a higher total volume (and thereby a higher dose) than is tolerable by multiple direct IM injections. Limb perfusion has been shown to be feasible in non-human primates using the rAAV1 serotype and a ubiquitous promoter expressing an epitope-tagged AAT matched to the host species.
Methods: In this study, we performed a biodistribution and preclinical safety study in non-human primates with a clinical candidate rAAV1-human AAT (hAAT) vector at doses ranging from 3.0 × 1012 to 1.3 × 1013 vg/kg, bracketing those used in our clinical trials.
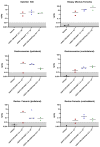
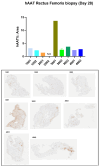
Results: We found that limb perfusion delivery of rAAV1-hAAT was safe and showed a biodistribution pattern similar to previous studies. However, serum levels of AAT obtained with high-dose limb perfusion still reached only ~50% of the target serum levels.
Conclusions: Our results suggest that clinically effective AAT gene therapy may ultimately require delivery at doses between 3.5 × 1013-1 × 1014 vg/kg, which is within the dose range used for approved rAAV gene therapies. Muscle-targeting strategies could be incorporated when delivering systemic administration of high-dose rAAV gene therapies to increase transduction of muscle tissues and reduce the burden on the liver, especially in diseases that can present with hepatotoxicity such as AAT deficiency.
Keywords: AATD gene therapy; alpha-1 anti-trypsin; biodistribution; pre-clinical study; rAAV gene therapy; rAAV safety.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Zieger M., Borel F., Greer C., Gernoux G., Blackwood M., Flotte T.R., Mueller C. Liver-directed serpina1 gene therapy attenuates progression of spontaneous and tobacco smoke-induced emphysema in alpha1-antitrypsin null mice. Mol. Ther. Methods Clin. Dev. 2022;25:425–438. doi: 10.1016/j.omtm.2022.04.003. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

