Duration of Dual Antiplatelet Therapy after Percutaneous Coronary Intervention of Unprotected Left Main Coronary Artery Stenosis: 6 versus 12 Months
- PMID: 39336936
- PMCID: PMC11431983
- DOI: 10.3390/jcm13185449
Duration of Dual Antiplatelet Therapy after Percutaneous Coronary Intervention of Unprotected Left Main Coronary Artery Stenosis: 6 versus 12 Months
Abstract
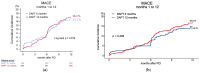
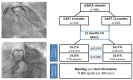
Background/Objectives: For patients with percutaneous coronary intervention (PCI) of an unprotected left main coronary artery (uLMCA) stenosis, the optimal duration of dual antiplatelet therapy (DAPT) remains a matter of debate. The purpose of this study was to compare clinical outcomes of 6- versus 12-month DAPT duration in patients with PCI of an uLMCA and stable angina. Methods: In this retrospective analysis, we included consecutive patients of our centre who underwent PCI of uLMCA stenosis for stable angina and who received DAPT with acetylsalicylic acid and clopidogrel for either 6 or 12 months. The primary endpoint was the composite of all-cause mortality, myocardial infarction, and target lesion revascularization at one year. Secondary endpoints included individual components of the primary endpoint, definite/probable stent thrombosis, and bleeding. Clinical outcomes were assessed by unadjusted analysis and by inverse probability of treatment weighting (IPTW). Results: Out of 984 included patients, 339 (34.5%) received DAPT for 6 months and 645 (65.5%) for 12 months. The primary endpoint occurred in 51 patients (15.2%) in the 6-month group and in 104 (16.3%) in the 12-month group (p = 0.674). Incidences of stent thrombosis (0.9% versus 0.3%, p = 0.224) and BARC 3,4,5 bleeding (6% versus 5.8%, p = 0.808) were also comparable in both groups. We found no significant differences in the primary endpoint and its components or BARC 3,4,5 bleeding between 6 and 12 months. Conclusions: Our findings do not support the extension of DAPT beyond 6 months after PCI for uLMCA in patients with stable angina.
Keywords: coronary artery disease; drug-eluting stents; dual antiplatelet therapy; left main disease; percutaneous coronary intervention.
Conflict of interest statement
T.H. has received travel grants from Boston Scientific and Novartis. D.W. reports personal fees from Abiomed, AstraZeneca, Bayer, Boehringer Ingelheim, Medtronic, and Novartis. F.-J.N. reports speaker honoraria from Amgen, Boston Scientific, and Daiichi Sankyo and consultancy fees from Novartis and Meril as well as speaker honoraria paid to his institution from Ferrer and Amgen, in addition to research grants from Bayer Healthcare, Boston Scientific, Biotronic, Edwards Lifesciences, Abbott, and Medtronic. P.B. reports personal fees from AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Novo Nordisk, Pfizer, and Sanofi Aventis. K.F. has received speakers fees from Astra Zeneca, Novartis, and Bayer; training grants from Boston Scientific and Orbus Neich; and research support from Daiichi Sankyo. The remaining co-authors have no conflicts of interest to declare.
Figures

References
-
- Buszman P.E., Buszman P.P., Kiesz R.S., Bochenek A., Trela B., Konkolewska M., Wallace-Bradley D., Wilczynski M., Banasiewicz-Szkrobka I., Peszek-Przybyla E., et al. Early and long-term results of unprotected left main coronary artery stenting: The LE MANS (Left Main Coronary Artery Stenting) registry. J. Am. Coll. Cardiol. 2009;54:1500–1511. doi: 10.1016/j.jacc.2009.07.007. - DOI - PubMed
-
- Khan S.U., Singh M., Valavoor S., Khan M.U., Lone A.N., Khan M.Z., Khan M.S., Mani P., Kapadia S.R., Michos E.D., et al. Dual Antiplatelet Therapy After Percutaneous Coronary Intervention and Drug-Eluting Stents: A Systematic Review and Network Meta-Analysis. Circulation. 2020;142:1425–1436. doi: 10.1161/CIRCULATIONAHA.120.046308. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

