Every Third Male Patient with Acromegaly Recovers from Hypogonadism after Neurosurgical Treatment
- PMID: 39337013
- PMCID: PMC11432164
- DOI: 10.3390/jcm13185526
Every Third Male Patient with Acromegaly Recovers from Hypogonadism after Neurosurgical Treatment
Abstract
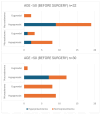
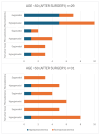
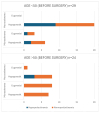
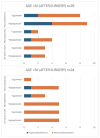
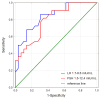
Background: Acromegaly is a rare endocrine condition caused by excessive growth hormone (GH) production. Hypogonadotropic hypogonadism (HH) affects 30%-50% of acromegaly patients. Objectives: This study examined the frequency of HH in men with acromegaly and the effects of neurosurgical treatment during the follow-up period. Materials and Methods: A retrospective analysis of medical records from January 2015 to December 2022 was conducted. Data included clinical history, laboratory results, and pituitary MRI findings. Statistical analysis was performed using Statistica 13.3. Results: Patients were divided into two groups: a cross-sectional sample (preoperative n = 62; postoperative n = 60) and a longitudinal sample (n = 53). In the longitudinal sample, preoperative HH was diagnosed in 41 males (77.36%). Post-surgery, HH prevalence decreased to 58.49% (n = 31), with a significant increase in postoperative testosterone levels (9.1 vs. 12.1 nmol/L; p < 0.001), particularly in patients with preoperative HH (7.2 vs. 10.2 nmol/L; p < 0.001). Among 41 patients with HH, 12 (29.27%) showed recovery. Testosterone levels were lower in patients with macroadenomas (7.2 nmol/L vs. 11.05 nmol/L; p < 0.001). Patients with HH had higher baseline levels of GH and insulin-like growth factor 1 (IGF-1) (GH: 3.37 ng/mL; IGF-1: 551 ng/mL vs. GH: 1.36 ng/mL; IGF-1: 355 ng/mL). Luteinizing hormone (LH) levels above 3.3 mIU/mL and follicle-stimulating hormone (FSH) levels above 4.4 mIU/mL predicted hypogonadism remission (Area under the curve (AUC): 0.838 and 0.792, respectively). Conclusions: Younger patients with macroadenoma and hyperprolactinemia are more likely to have preoperative hypogonadism. Neurosurgical treatment can normalize LH, FSH, and total testosterone in approximately 30% of these patients.
Keywords: GH; acromegaly; hypogonadotropic hypogonadism; pituitary adenoma; pituitary tumor; testosterone.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

