Neutrophil Extracellular Traps Affect Human Inner Ear Vascular Permeability
- PMID: 39337254
- PMCID: PMC11431685
- DOI: 10.3390/ijms25189766
Neutrophil Extracellular Traps Affect Human Inner Ear Vascular Permeability
Abstract
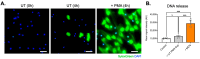
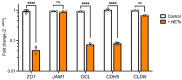
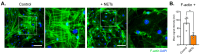
The integrity of the blood-labyrinth barrier (BLB) is essential for inner ear homeostasis, regulating the ionic composition of endolymph and perilymph and preventing harmful substance entry. Endothelial hyperpermeability, central in inflammatory and immune responses, is managed through complex intercellular communication and molecular signaling pathways. Recent studies link BLB permeability dysregulation to auditory pathologies like acoustic trauma, autoimmune inner ear diseases, and presbycusis. Polymorphonuclear granulocytes (PMNs), or neutrophils, significantly modulate vascular permeability, impacting endothelial barrier properties. Neutrophil extracellular traps (NETs) are involved in diseases with autoimmune and autoinflammatory bases. The present study evaluated the impact of NETs on a BLB cellular model using a Transwell® setup. Our findings revealed a concentration-dependent impact of NETs on human inner ear-derived endothelial cells. In particular, endothelial permeability markers increased, as indicated by reduced transepithelial electrical resistance, enhanced dextran permeability, and downregulated junctional gene expression (ZO1, OCL, and CDH5). Changes in cytoskeletal architecture were also observed. These preliminary results pave the way for further research into the potential involvement of NETs in BLB impairment and implications for auditory disorders.
Keywords: Meniere’s disease; TEER; blood–labyrinth barrier; hearing loss; tissue model.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

