Comparison of Mitochondrial and Antineoplastic Effects of Amiodarone and Desethylamiodarone in MDA-MB-231 Cancer Line
- PMID: 39337269
- PMCID: PMC11432025
- DOI: 10.3390/ijms25189781
Comparison of Mitochondrial and Antineoplastic Effects of Amiodarone and Desethylamiodarone in MDA-MB-231 Cancer Line
Abstract
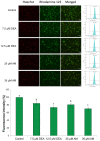
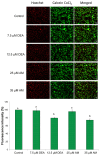
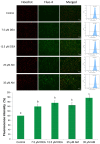
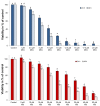
Previously, we have demonstrated that amiodarone (AM), a widely used antiarrhythmic drug, and its major metabolite desethylamiodarone (DEA) both affect several mitochondrial processes in isolated heart and liver mitochondria. Also, we have established DEA's antitumor properties in various cancer cell lines and in a rodent metastasis model. In the present study, we compared AM's and DEA's mitochondrial and antineoplastic effects in a human triple-negative breast cancer (TNBC) cell line. Both compounds reduced viability in monolayer and sphere cultures and the invasive growth of the MDA-MB-231 TNBC line by inducing apoptosis. They lowered mitochondrial trans-membrane potential, increased Ca2+ influx, induced mitochondrial permeability transition, and promoted mitochondrial fragmentation. In accordance with their mitochondrial effects, both substances massively decreased overall, and even to a greater extent, mitochondrial ATP production decreased, as determined using a Seahorse live cell respirometer. In all these effects, DEA was more effective than AM, indicating that DEA may have higher potential in the therapy of TNBC than its parent compound.
Keywords: apoptosis; cancer cell energy metabolism; drug repositioning; mitochondrial drug; mitochondrial dysfunction.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; the collection, analyses, or interpretation of data; the writing of the manuscript, or the decision to publish the results.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

