Hypoxia in Human Obesity: New Insights from Inflammation towards Insulin Resistance-A Narrative Review
- PMID: 39337290
- PMCID: PMC11432683
- DOI: 10.3390/ijms25189802
Hypoxia in Human Obesity: New Insights from Inflammation towards Insulin Resistance-A Narrative Review
Abstract
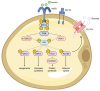
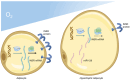
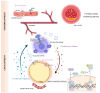
Insulin resistance (IR), marked by reduced cellular responsiveness to insulin, and obesity, defined by the excessive accumulation of adipose tissue, are two intertwined conditions that significantly contribute to the global burden of cardiometabolic diseases. Adipose tissue, beyond merely storing triglycerides, acts as an active producer of biomolecules. In obesity, as adipose tissue undergoes hypertrophy, it becomes dysfunctional, altering the release of adipocyte-derived factors, known as adipokines. This dysfunction promotes low-grade chronic inflammation, exacerbates IR, and creates a hyperglycemic, proatherogenic, and prothrombotic environment. However, the fundamental cause of these phenomena remains unclear. This narrative review points to hypoxia as a critical trigger for the molecular changes associated with fat accumulation, particularly within visceral adipose tissue (VAT). The activation of hypoxia-inducible factor-1 (HIF-1), a transcription factor that regulates homeostatic responses to low oxygen levels, initiates a series of molecular events in VAT, leading to the aberrant release of adipokines, many of which are still unexplored, and potentially affecting peripheral insulin sensitivity. Recent discoveries have highlighted the role of hypoxia and miRNA-128 in regulating the insulin receptor in visceral adipocytes, contributing to their dysfunctional behavior, including impaired glucose uptake. Understanding the complex interplay between adipose tissue hypoxia, dysfunction, inflammation, and IR in obesity is essential for developing innovative, targeted therapeutic strategies.
Keywords: adipokine; adipose tissue; hypoxia; inflammation; insulin resistance; micro-RNA.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Root H.F. Insulin Resistance and Bronze Diabetes. N. Engl. J. Med. 1929;201:201–206. doi: 10.1056/NEJM192908012010501. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

