The Drug Transporter P-Glycoprotein and Its Impact on Ceramide Metabolism-An Unconventional Ally in Cancer Treatment
- PMID: 39337312
- PMCID: PMC11432138
- DOI: 10.3390/ijms25189825
The Drug Transporter P-Glycoprotein and Its Impact on Ceramide Metabolism-An Unconventional Ally in Cancer Treatment
Abstract
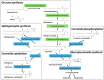
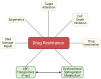
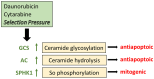
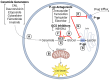
The tumor-suppressor sphingolipid ceramide is recognized as a key participant in the cytotoxic mechanism of action of many types of chemotherapy drugs, including anthracyclines, Vinca alkaloids, the podophyllotoxin etoposide, taxanes, and the platinum drug oxaliplatin. These drugs can activate de novo synthesis of ceramide or stimulate the production of ceramide via sphingomyelinases to limit cancer cell survival. On the contrary, dysfunctional sphingolipid metabolism, a prominent factor in cancer survival and therapy resistance, blunts the anticancer properties of ceramide-orchestrated cell death pathways, especially apoptosis. Although P-glycoprotein (P-gp) is famous for its role in chemotherapy resistance, herein, we propose alternate interpretations and discuss the capacity of this multidrug transporter as a "ceramide neutralizer", an unwelcome event, highlighting yet another facet of P-gp's versatility in drug resistance. We introduce sphingolipid metabolism and its dysfunctional regulation in cancer, present a summary of factors that contribute to chemotherapy resistance, explain how P-gp "neutralizes" ceramide by hastening its glycosylation, and consider therapeutic applications of the P-gp-ceramide connection in the treatment of cancer.
Keywords: P-glycoprotein; cancer drug resistance; ceramide; sphingolipids.
Conflict of interest statement
D.J.F. received research funding, honoraria, and/or stock options from AstraZeneca, Dren Bio, Recludix Pharma, and Kymera Therapeutics. T.P.L., Jr. received a scientific advisory board membership, consultancy fees, honoraria, and/or stock options from Keystone Nano, Flagship Labs 86, Dren Bio, Recludix Pharma, Kymera Therapeutics, and Prime Genomics. M.C.C. owns shares in Keystone Nano. The remaining authors declare that there are no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

