Polymorphism and Pharmacological Assessment of Carbamazepine
- PMID: 39337323
- PMCID: PMC11431949
- DOI: 10.3390/ijms25189835
Polymorphism and Pharmacological Assessment of Carbamazepine
Abstract
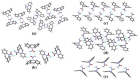
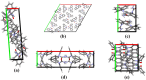
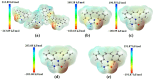
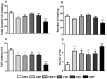
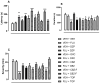
This work provides insight into carbamazepine polymorphs (Forms I, II, III, IV, and V), with reports on the cytoprotective, exploratory, motor, CNS-depressant, and anticonvulsant properties of carbamazepine (CBZ), carbamazepine formulation (CBZ-F), topiramate (TOP), oxcarbazepine (OXC), and diazepam (DZP) in mice. Structural analysis highlighted the significant difference in molecular conformations, which directly influence the physicochemical properties; and density functional theory description provided indications about CBZ reactivity and stability. In addition to neuron viability assessment in vitro, animals were treated orally with vehicle 10 mL/kg, as well as CBZ, CBZ-F, TOP, OXC, and DZP at the dose of 5 mg/kg and exposed to open-field, rotarod, barbiturate sleep induction and pentylenetetrazol (PTZ 70 mg/kg)-induced seizure. The involvement of GABAergic mechanisms in the activity of these drugs was evaluated with the intraperitoneal pretreatment of flumazenil (2 mg/kg). The CBZ, CBZ-F, and TOP mildly preserved neuronal viability. The CBZ-F and the reference AEDs potentiated barbiturate sleep, altered motor activities, and attenuated PTZ-induced convulsion. However, flumazenil pretreatment blocked these effects. Additional preclinical assessments could further establish the promising utility of CBZ-F in clinical settings while expanding the scope of AED formulations and designs.
Keywords: animal models; anticonvulsant; carbamazepine; cytoprotection; formulation; polymorphism.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


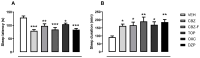
References
-
- Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., Hirsch E., Jain S., Mathern G.W., Moshé S.L., et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. - DOI - PMC - PubMed
-
- Marson A.G., Al-Kharusi A.M., Alwaidh M., Appleton R., Baker G.A., Chadwick D.W., Cramp C., Cockerell O.C., Cooper P.N., Doughty J., et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: An unblinded randomised controlled trial. Lancet. 2007;369:1000–1015. doi: 10.1016/S0140-6736(07)60460-7. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

