Photo-Crosslinked Pro-Angiogenic Hydrogel Dressing for Wound Healing
- PMID: 39337435
- PMCID: PMC11432402
- DOI: 10.3390/ijms25189948
Photo-Crosslinked Pro-Angiogenic Hydrogel Dressing for Wound Healing
Abstract
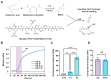
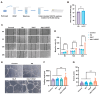
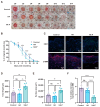
Severe burns are one of the most devastating injuries, in which sustained inflammation and ischemia often delay the healing process. Pro-angiogenic growth factors such as vascular endothelial growth factor (VEGF) have been widely studied for promoting wound healing. However, the short half-life and instability of VEGF limit its clinical applications. In this study, we develop a photo-crosslinked hydrogel wound dressing from methacrylate hyaluronic acid (MeHA) bonded with a pro-angiogenic prominin-1-binding peptide (PR1P). The materials were extruded in wound bed and in situ formed a wound dressing via exposure to short-time ultraviolet radiation. The study shows that the PR1P-bonded hydrogel significantly improves VEGF recruitment, tubular formation, and cell migration in vitro. Swelling, Scanning Electron Microscope, and mechanical tests indicate the peptide does not affect the overall mechanical and physical properties of the hydrogels. For in vivo studies, the PR1P-bonded hydrogel dressing enhances neovascularization and accelerates wound closure in both deep second-degree burn and full-thickness excisional wound models. The Western blot assay shows such benefits can be related to the activation of the VEGF-Akt signaling pathway. These results suggest this photo-crosslinked hydrogel dressing efficiently promotes VEGF recruitment and angiogenesis in skin regeneration, indicating its potential for clinical applications in wound healing.
Keywords: burn; hydrogel; methacrylate hyaluronic acid; prominin-1-binding peptide; vascular endothelial growth factor; wound healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

