A Snapshot of the Most Recent Transthyretin Stabilizers
- PMID: 39337457
- PMCID: PMC11432176
- DOI: 10.3390/ijms25189969
A Snapshot of the Most Recent Transthyretin Stabilizers
Abstract
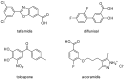
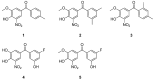
In recent years, several strategies have been developed for the treatment of transthyretin-related amyloidosis, whose complex clinical manifestations involve cardiomyopathy and polyneuropathy. In view of this, transthyretin stabilizers represent a major cornerstone in treatment thanks to the introduction of tafamidis into therapy and the entry of acoramidis into clinical trials. However, the clinical treatment of transthyretin-related amyloidosis still presents several challenges, urging the development of new and improved therapeutics. Bearing this in mind, in this paper, the most promising among the recently published transthyretin stabilizers were reviewed. Their activity was described to provide some insights into their clinical potential, and crystallographic data were provided to explain their modes of action. Finally, structure-activity relationship studies were performed to give some guidance to future researchers aiming to synthesize new transthyretin stabilizers. Interestingly, some new details emerged with respect to the previously known general rules that guided the design of new compounds.
Keywords: SAR; TTR; activity; binding affinity; crystallographic studies; stabilizers; structure–activity relationship studies; synthetic compounds; transthyretin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


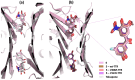
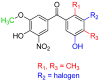
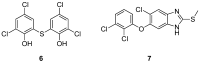
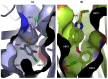

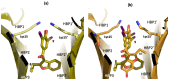

References
-
- Wojtczak A., Cody V., Luft J.R., Pangborn W. Structure of Rat Transthyretin (rTTR) Complex with Thyroxine at 2.5 Å Resolution: First Non-Biased Insight into Thyroxine Binding Reveals Different Hormone Orientation in Two Binding Sites. Acta Crystallogr. D Biol. Crystallogr. 2001;57:1061–1070. doi: 10.1107/S0907444901007235. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

