Cartilage Repair: Promise of Adhesive Orthopedic Hydrogels
- PMID: 39337473
- PMCID: PMC11432485
- DOI: 10.3390/ijms25189984
Cartilage Repair: Promise of Adhesive Orthopedic Hydrogels
Abstract
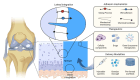
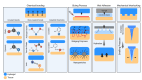
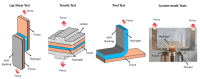
Cartilage repair remains a major challenge in human orthopedic medicine, necessitating the application of innovative strategies to overcome existing technical and clinical limitations. Adhesive hydrogels have emerged as promising candidates for cartilage repair promotion and tissue engineering, offering key advantages such as enhanced tissue integration and therapeutic potential. This comprehensive review navigates the landscape of adhesive hydrogels in cartilage repair, discussing identified challenges, shortcomings of current treatment options, and unique advantages of adhesive hydrogel products and scaffolds. While emphasizing the critical need for in situ lateral integration with surrounding tissues, we dissect current limitations and outline future perspectives for hydrogel scaffolds in cartilage repair. Moreover, we examine the clinical translation pathway and regulatory considerations specific to adhesive hydrogels. Overall, this review synthesizes the existing insights and knowledge gaps and highlights directions for future research regarding adhesive hydrogel-based devices in advancing cartilage tissue engineering.
Keywords: ACI; adhesion; carrier; cartilage; delivery; hydrogel; integration; therapeutics.
Conflict of interest statement
Author A.L. was employed by LAM Biotechnologies SA (Epalinges, Switzerland) during this work. The remaining authors declare no conflicts of interest in relation with this work.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

