Chondroitin Sulfate for Cartilage Regeneration, Administered Topically Using a Nanostructured Formulation
- PMID: 39337510
- PMCID: PMC11432425
- DOI: 10.3390/ijms251810023
Chondroitin Sulfate for Cartilage Regeneration, Administered Topically Using a Nanostructured Formulation
Abstract
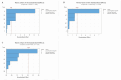
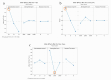
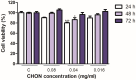
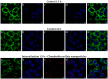
In the pharmaceutical sector, solid lipid nanoparticles (SLN) are vital for drug delivery incorporating a lipid core. Chondroitin sulfate (CHON) is crucial for cartilage health. It is often used in osteoarthritis (OA) treatment. Due to conflicting results from clinical trials on CHON's efficacy in OA treatment, there has been a shift toward exploring effective topical systems utilizing nanotechnology. This study aimed to optimize a solid lipid nanoparticle formulation aiming to enhance CHON permeation for OA therapy. A 3 × 3 × 2 Design of these experiments determined the ideal parameters: a CHON concentration of 0.4 mg/mL, operating at 20,000 rpm speed, and processing for 10 min for SLN production. Transmission electron microscopy analysis confirmed the nanoparticles' spherical morphology, ensuring crucial uniformity for efficient drug delivery. Cell viability assessments showed no significant cytotoxicity within the tested parameters, indicating a safe profile for potential clinical application. The cell internalization assay indicates successful internalization at 1.5 h and 24 h post-treatment. Biopharmaceutical studies supported SLNs, indicating them to be effective CHON carriers through the skin, showcasing improved skin permeation and CHON retention compared to conventional methods. In summary, this study successfully optimized SLN formulation for efficient CHON transport through pig ear skin with no cellular toxicity, highlighting SLNs' potential as promising carriers to enhance CHON delivery in OA treatment and advance nanotechnology-based therapeutic strategies in pharmaceutical formulations.
Keywords: biopharmaceutical studies; cell viability; chondroitin sulfate; design of experiments; drug delivery; osteoarthritis; skin permeation; solid lipid nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Pund S., Joshi A. Nanoarchitectures for Neglected Tropical Protozoal Diseases: Challenges and State of the Art. In: Grumezescu A.M., editor. Nano- and Microscale Drug Delivery Systems: Design and Fabrication. 1st ed. Elsevier; Amsterdam, The Netherlands: 2017. p. 449.
-
- Gasco M.R. Method for producing solid lipid microspheres having a narrow size distribution. No. 5,250,236. U.S. Patent. 1993 October 5;
-
- Villafuerte L., García B., de Lourdes Garzón M., Hernández A., Vázquez M.L. Nanopartículas lipídicas sólidas. [(accessed on 23 November 2023)];Rev. Mex. Cienc. Farm. [En Linea] 2008 39:38–52. Available online: https://www.redalyc.org/pdf/579/57939107.pdf.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

