Polyamine Pathway Inhibitor DENSPM Suppresses Lipid Metabolism in Pheochromocytoma Cell Line
- PMID: 39337514
- PMCID: PMC11432427
- DOI: 10.3390/ijms251810029
Polyamine Pathway Inhibitor DENSPM Suppresses Lipid Metabolism in Pheochromocytoma Cell Line
Abstract
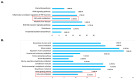
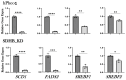
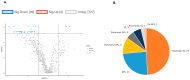
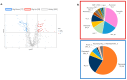
Pheochromocytomas (PCCs) are tumors arising from chromaffin cells in the adrenal medulla, and paragangliomas (PGLs) are tumors derived from extra-adrenal sympathetic or parasympathetic paraganglia; these tumors are collectively referred to as PPGL cancer. Treatment for PPGL primarily involves surgical removal of the tumor, and only limited options are available for treatment of the disease once it becomes metastatic. Human carriers of the heterozygous mutations in the succinate dehydrogenase subunit B (SDHB) gene are susceptible to the development of PPGL. A physiologically relevant PCC patient-derived cell line hPheo1 was developed, and SDHB_KD cells carrying a stable short hairpin knockdown of SDHB were derived from it. An untargeted metabolomic approach uncovered an overactive polyamine pathway in the SDHB_KD cells that was subsequently fully validated in a large set of human SDHB-mutant PPGL tumor samples. We previously reported that treatment with the polyamine metabolism inhibitor N1,N11-diethylnorspermine (DENSPM) drastically inhibited growth of these PCC-derived cells in culture as well as in xenograft mouse models. Here we explored the mechanisms underlying DENSPM action in hPheo1 and SDHB_KD cells. Specifically, by performing an RNAseq analysis, we have identified gene expression changes associated with DENSPM treatment that broadly interfere with all aspects of lipid metabolism, including fatty acid (FA) synthesis, desaturation, and import/uptake. Furthermore, by performing an untargeted lipidomic liquid chromatography-mass spectrometry (LC/MS)-based analysis we uncovered specific groups of lipids that are dramatically reduced as a result of DENSPM treatment. Specifically, the bulk of plasmanyl ether lipid species that have been recently reported as the major determinants of cancer cell fate are notably decreased. In summary, this work suggests an intersection between active polyamine and lipid pathways in PCC cells.
Keywords: DENSPM; SDHB; paraganglioma; pheochromocytoma; plasmanyl.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Taïeb D., Wanna G.B., Ahmad M., Lussey-Lepoutre C., Perrier N.D., Nölting S., Amar L., Timmers H.J.L.M., Schwam Z.G., Estrera A.L., et al. Clinical consensus guideline on the management of phaeochromocytoma and paraganglioma in patients harbouring germline SDHD pathogenic variants. Lancet Diabetes Endocrinol. 2023;11:345–361. doi: 10.1016/S2213-8587(23)00038-4. - DOI - PMC - PubMed
-
- Fishbein L., Leshchiner I., Walter V., Danilova L., Robertson A.G., Johnson A.R., Lichtenberg T.M., Murray B.A., Ghayee H.K., Else T., et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell. 2017;31:181–193. doi: 10.1016/j.ccell.2017.01.001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

