Mobilization of Endogenous CD34+/CD133+ Endothelial Progenitor Cells by Enhanced External Counter Pulsation for Treatment of Refractory Angina
- PMID: 39337516
- PMCID: PMC11432706
- DOI: 10.3390/ijms251810030
Mobilization of Endogenous CD34+/CD133+ Endothelial Progenitor Cells by Enhanced External Counter Pulsation for Treatment of Refractory Angina
Abstract
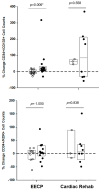
Adult stem cell therapy via intramyocardial injection of autologous CD34+ stem cells has been shown to improve exercise capacity and reduce angina frequency and mortality in patients with refractory angina (RA). However, the cost of such therapy is a limitation to its adoption in clinical practice. Our goal was to determine whether the less costly, less invasive, and widely accessible, FDA-approved alternative treatment for RA patients, known as enhanced external counterpulsation (EECP), mobilizes endogenous CD34+ stem cells and whether such mobilization is associated with the clinical benefits seen with intramyocardial injection. We monitored changes in circulating levels of CD34+/CD133+ and CD34+/KDR+ cells in RA patients undergoing EECP therapy and in a comparator cohort of RA patients undergoing an exercise regimen known as cardiac rehabilitation. Changes in exercise capacity in both cohorts were monitored by measuring treadmill times (TT), double product (DP) scores, and Canadian Cardiovascular Society (CCS) angina scores between pre- and post-treatment treadmill stress tests. Circulating levels of CD34+/CD133+ cells increased in patients undergoing EECP and were significant (β = -2.38, p = 0.012) predictors of improved exercise capacity in these patients. CD34+/CD133+ cells isolated from RA patients could differentiate into endothelial cells, and their numbers increased during EECP therapy. Our results support the hypothesis that mobilized CD34+/CD133+ cells repair vascular damage and increase collateral circulation in RA patients. They further support clinical interventions that can mobilize adult CD34+ stem cells as therapy for patients with RA and other vascular diseases.
Keywords: adult stem cells; endothelial progenitor cells (EPCs); endothelial-to-hematopoietic stem cell (HSC) transition; enhanced external counterpulsation (EECP); ischemic coronary artery disease; refractory angina (RA).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. - DOI - PMC - PubMed
-
- Henry T.D., Losordo D.W., Traverse J.H., Schatz R.A., Jolicoeur E.M., Schaer G.L., Clare R., Chiswell K., White C.J., Fortuin F.D., et al. Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option refractory angina: A patient-level pooled analysis of randomized double-blinded trials. Eur. Heart J. 2018;39:2208–2216. doi: 10.1093/eurheartj/ehx764. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

