OXGR1-Dependent (Pro)Renin Receptor Upregulation in Collecting Ducts of the Clipped Kidney Contributes to Na+ Balance in Goldblatt Hypertensive Mice
- PMID: 39337535
- PMCID: PMC11432382
- DOI: 10.3390/ijms251810045
OXGR1-Dependent (Pro)Renin Receptor Upregulation in Collecting Ducts of the Clipped Kidney Contributes to Na+ Balance in Goldblatt Hypertensive Mice
Abstract
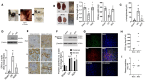
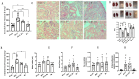
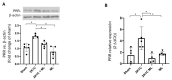
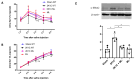
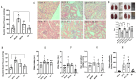
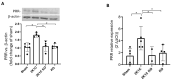
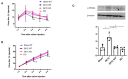
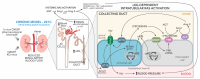
The two-kidney, one-clip (2K1C) Goldblatt rodent model elicits a reduction in renal blood flow (RBF) in the clipped kidney (CK). The reduced RBF and oxygen bio-ability causes the accumulation of the tricarboxylic cycle intermediary, α-ketoglutarate, which activates the oxoglutarate receptor-1 (OXGR1). In the kidney, OXGR1 is abundantly expressed in intercalated cells (ICs) of the collecting duct (CD), thus contributing to sodium transport and electrolyte balance. The (pro)renin receptor (PRR), a member of the renin-angiotensin system (RAS), is a key regulator of sodium reabsorption and blood pressure (BP) that is expressed in ICs. The PRR is upregulated in 2K1C rats. Here, we tested the hypothesis that chronic reduction in RBF in the CK leads to OXGR1-dependent PRR upregulation in the CD and alters sodium balance and BP in 2K1C mice. To determine the role of OXGR1 in regulating the PRR in the CDs during renovascular hypertension, we performed 2K1C Goldblatt surgery (clip = 0.13 mm internal gap, 14 days) in two groups of male mice: (1) mice treated with Montelukast (OXGR1 antagonist; 5 mg/Kg/day); (2) OXGR1-/- knockout mice. Wild-type and sham-operated mice were used as controls. After 14 days, 2K1C mice showed increased systolic BP (SBP) (108 ± 11 vs. control 82 ± 5 mmHg, p < 0.01) and a lower natriuretic response after the saline challenge test. The CK group showed upregulation of erythropoietin, augmented α-ketoglutarate, and increased PRR expression in the renal medulla. The CK of OXGR1 knockout mice and mice subjected to the OXGR1 antagonist elicited impaired PRR upregulation, attenuated SBP, and better natriuretic responses. In 2K1C mice, the effect of reduced RBF on the OXGR1-dependent PRR upregulation in the CK may contribute to the anti-natriuretic and increased SBP responses.
Keywords: 2K1C; kidney; prorenin; renal blood flow; tricarboxylic cyclic acid pathway.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Nurun N.A.H.M., Uddin N.M., Nakagawa T., Iwata H., Ichihara A., Inagami T., Suzuki F. Role of “handle” region of prorenin prosegment in the non-proteolytic activation of prorenin by binding to membrane anchored (pro)renin receptor. Front. Biosci-Landmrk. 2007;12:4810–4817. doi: 10.2741/2429. - DOI - PubMed
-
- Redublo Quinto B.M., Camargo de Andrade M.C., Ronchi F.A., Santos E.L., Alves Correa S.A., Shimuta S.I., Pesquero J.B., Mortara R.A., Casarini D.E. Expression of angiotensin I-converting enzymes and bradykinin B-2 receptors in mouse inner medullary-collecting duct cells. Int. Immunopharmacol. 2008;8:254–260. doi: 10.1016/j.intimp.2007.09.013. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

