Characterization of Neutrophil Functional Responses to SARS-CoV-2 Infection in a Translational Feline Model for COVID-19
- PMID: 39337543
- PMCID: PMC11432149
- DOI: 10.3390/ijms251810054
Characterization of Neutrophil Functional Responses to SARS-CoV-2 Infection in a Translational Feline Model for COVID-19
Abstract
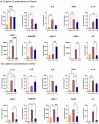
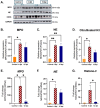
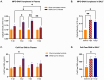
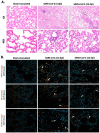
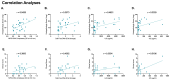
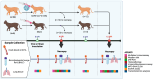
There is a complex interplay between viral infection and host innate immune response regarding disease severity and outcomes. Neutrophil hyperactivation, including excessive release of neutrophil extracellular traps (NETs), is linked to exacerbated disease in acute COVID-19, notably in hospitalized patients. Delineating protective versus detrimental neutrophil responses is essential to developing targeted COVID-19 therapies and relies on high-quality translational animal models. In this study, we utilize a previously established feline model for COVID-19 to investigate neutrophil dysfunction in which experimentally infected cats develop clinical disease that mimics acute COVID-19. Specific pathogen-free cats were inoculated with SARS-CoV-2 (B.1.617.2; Delta variant) (n = 24) or vehicle (n = 6). Plasma, bronchoalveolar lavage fluid, and lung tissues were collected at various time points over 12 days post-inoculation. Systematic and temporal evaluation of the kinetics of neutrophil activation was conducted by measuring markers of activation including myeloperoxidase (MPO), neutrophil elastase (NE), and citrullinated histone H3 (citH3) in SARS-CoV-2-infected cats at 4 and 12 days post-inoculation (dpi) and compared to vehicle-inoculated controls. Cytokine profiling supported elevated innate inflammatory responses with specific upregulation of neutrophil activation and NET formation-related markers, namely IL-8, IL-18, CXCL1, and SDF-1, in infected cats. An increase in MPO-DNA complexes and cell-free dsDNA in infected cats compared to vehicle-inoculated was noted and supported by histopathologic severity in respiratory tissues. Immunofluorescence analyses further supported correlation of NET markers with tissue damage, especially 4 dpi. Differential gene expression analyses indicated an upregulation of genes associated with innate immune and neutrophil activation pathways. Transcripts involved in activation and NETosis pathways were upregulated by 4 dpi and downregulated by 12 dpi, suggesting peak activation of neutrophils and NET-associated markers in the early acute stages of infection. Correlation analyses conducted between NET-specific markers and clinical scores as well as histopathologic scores support association between neutrophil activation and disease severity during SARS-CoV-2 infection in this model. Overall, this study emphasizes the effect of neutrophil activation and NET release in SARS-CoV-2 infection in a feline model, prompting further investigation into therapeutic strategies aimed at mitigating excessive innate inflammatory responses in COVID-19.
Keywords: COVID-19; SARS-CoV-2; feline; neutrophil; neutrophil extracellular traps.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Aleem A., Akbar Samad A.B., Vaqar S. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2021. Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19) - PubMed
-
- Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2020. Features, evaluation, and treatment of coronavirus (COVID-19) - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

