Development and Validation of the MAST ISOPLEX®VTEC Kit for Simultaneous Detection of Shiga Toxin/Verotoxin 1 and 2 (stx1/vt1 and stx2/vt2) with Inhibition Control (IC) in a Rapid Loop-Mediated Isothermal Amplification (LAMP) Multiplex Assay
- PMID: 39337553
- PMCID: PMC11432264
- DOI: 10.3390/ijms251810067
Development and Validation of the MAST ISOPLEX®VTEC Kit for Simultaneous Detection of Shiga Toxin/Verotoxin 1 and 2 (stx1/vt1 and stx2/vt2) with Inhibition Control (IC) in a Rapid Loop-Mediated Isothermal Amplification (LAMP) Multiplex Assay
Abstract
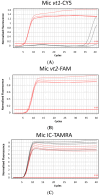
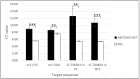
Loop-mediated isothermal amplification (LAMP) is a cost-effective, rapid, and highly specific method of replicating nucleic acids. Adding multiple targets into a single LAMP assay to create a multiplex format is highly desirable for clinical applications but has been challenging due to a need to develop specific detection techniques and strict primer design criteria. This study describes the evaluation of a rapid triplex LAMP assay, MAST ISOPLEX®VTEC, for the simultaneous detection of Shiga toxin/verotoxin 1 and 2 (stx1/vt1 and stx2/vt2) genes in verotoxigenic Escherichia coli (E. coli) (VTEC) isolates with inhibition control (IC) synthetic DNA using a single fluorophore-oligonucleotide probe, MAST ISOPLEX®Probes, integrated into the primer set of each target. MAST ISOPLEX®Probes used in the MAST ISOPLEX®VTEC kit produce fluorescent signals as they integrate with reaction products specific to each target, allowing tracking of multiple amplifications in real time using a real-time analyzer. Initial validation on DNA extracts from fecal cultures and synthetic DNA sequences (gBlocks) showed that the MAST ISOPLEX®VTEC kit provides a method for sensitive simultaneous triplex detection in a single assay with a limit of detection (LOD) of less than 100 target copies/assay and 96% and 100% sensitivity and specificity, respectively.
Keywords: DNA/LYO3; MAST ISOPLEX® VTEC; POC; loop-mediated isothermal amplification; lyophilization; multiplex.
Conflict of interest statement
Author Matthew Bennett was employed by the company Mast Group Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

