Molecular Aspects of the Interactions between Selected Benzodiazepines and Common Adulterants/Diluents: Forensic Application of Theoretical Chemistry Methods
- PMID: 39337573
- PMCID: PMC11432270
- DOI: 10.3390/ijms251810087
Molecular Aspects of the Interactions between Selected Benzodiazepines and Common Adulterants/Diluents: Forensic Application of Theoretical Chemistry Methods
Abstract
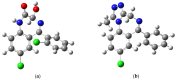
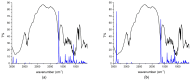
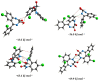
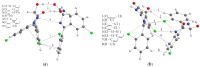
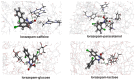
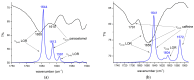
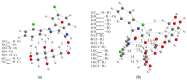
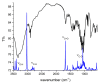
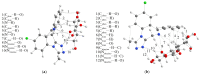
Benzodiazepines are frequently encountered in crime scenes, often mixed with adulterants and diluents, complicating their analysis. This study investigates the interactions between two benzodiazepines, lorazepam (LOR) and alprazolam (ALP), with common adulterants/diluents (paracetamol, caffeine, glucose, and lactose) using infrared (IR) spectroscopy and quantum chemical methods. The crystallographic structures of LOR and ALP were optimized using several functionals (B3LYP, B3LYP-D3BJ, B3PW91, CAM-B3LYP, M05-2X, and M06-2X) combined with the 6-311++G(d,p) basis set. M05-2X was the most accurate when comparing experimental and theoretical bond lengths and angles. Vibrational and 13C NMR spectra were calculated to validate the functional's applicability. The differences between LOR's experimental and theoretical IR spectra were attributed to intramolecular interactions between LOR monomers, examined through density functional theory (DFT) optimization and quantum theory of atoms in molecules (QTAIM) analysis. Molecular dynamics simulations modeled benzodiazepine-adulterant/diluent systems, predicting the most stable structures, which were further analyzed using QTAIM. The strongest interactions and their effects on IR spectra were identified. Comparisons between experimental and theoretical spectra confirmed spectral changes due to interactions. This study demonstrates the potential of quantum chemical methods in analyzing complex mixtures, elucidating spectral changes, and assessing the structural stability of benzodiazepines in forensic samples.
Keywords: DFT; IR; QTAIM; adulterants; benzodiazepines; psychoactive substances.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Verma S., Kumar S. A Mini Review on Synthetic Approaches and Biological Activities of Benzodiazepines. Mini. Rev. Org. Chem. 2017;14:453–468. doi: 10.2174/1570193X14666170511121927. - DOI
-
- Moosmann B., Auwärter V. New Psychoactive Substances. Handbook of Experimental Pharmacology. Springer; Berlin/Heidelberg, Germany: 2018. Designer Benzodiazepines: Another Class of New Psychoactive Substances; pp. 383–410. - PubMed
-
- dos Santos C., Bruni A.T. Evaluation of Density Functional Theory-Generated Data for Infrared Spectroscopy of Novel Psychoactive Substances Using Unsupervised Learning. Psychoactives. 2024;3:265–284. doi: 10.3390/psychoactives3020017. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

