Antimicrobial Activity of Arthrospira platensis-Mediated Gold Nanoparticles against Streptococcus pneumoniae: A Metabolomic and Docking Study
- PMID: 39337576
- PMCID: PMC11432420
- DOI: 10.3390/ijms251810090
Antimicrobial Activity of Arthrospira platensis-Mediated Gold Nanoparticles against Streptococcus pneumoniae: A Metabolomic and Docking Study
Abstract
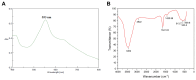
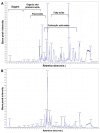
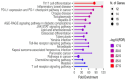
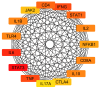
The emergence of antibiotic-resistant Streptococcus pneumoniae necessitates the discovery of novel therapeutic agents. This study investigated the antimicrobial potential of green-synthesized gold nanoparticles (AuNPs) fabricated using Arthrospira platensis extract. Characterization using Fourier transform infrared spectroscopy revealed the presence of functional groups such as ketones, aldehydes, and carboxylic acids in the capping agents, suggesting their role in AuNP stabilization. Transmission electron microscopy demonstrated the formation of rod-shaped AuNPs with a mean diameter of 134.8 nm, as determined by dynamic light scattering, and a zeta potential of -27.2 mV, indicating good colloidal stability. The synthesized AuNPs exhibited potent antibacterial activity against S. pneumoniae, with a minimum inhibitory concentration (MIC) of 12 μg/mL, surpassing the efficacy of the control antibiotic, tigecycline. To elucidate the underlying mechanisms of action, an untargeted metabolomic analysis of the A. platensis extract was performed, identifying 26 potential bioactive compounds belonging to diverse chemical classes. In silico studies focused on molecular docking simulations revealed that compound 22 exhibited a strong binding affinity to S. pneumoniae topoisomerase IV, a critical enzyme for bacterial DNA replication. Molecular dynamics simulations further validated the stability of this protein-ligand complex. These findings collectively highlight the promising antimicrobial potential of A. platensis-derived AuNPs and their constituent compounds, warranting further investigation for the development of novel anti-pneumococcal therapeutics.
Keywords: Arthrospira platensis; Streptococcus pneumoniae; antimicrobial; gold nanoparticles; metabolics; molecular docking; protein-protein interaction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Gierke R., Wodi A.P., Kobayashi M. Epidemiology and Prevention of Vaccine-Preventable Diseases. Centers for Disease Control and Prevention; Atlanta, GE, USA: 2021. Pneumococcal disease; pp. 279–296.
-
- Sihvonen R., Siira L., Toropainen M., Kuusela P., Pätäri-Sampo A. Streptococcus pneumoniae antimicrobial resistance decreased in the Helsinki Metropolitan Area after routine 10-valent pneumococcal conjugate vaccination of infants in Finland. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:2109–2116. doi: 10.1007/s10096-017-3033-5. - DOI - PubMed
-
- Örtqvist Å., Hedlund J., Kalin M. Seminars in Respiratory and Critical Care Medicine. Thieme Medical Publishers, Inc.; New York, NY, USA: 2005. Streptococcus pneumoniae: Epidemiology, risk factors, and clinical features. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- (NRF-2021R1A5A8032895)/This work was also supported by the National Research Foundation of Korea (NRF) grants funded by the Korean Ministry of Science and ICT (MSIT)
- (RSP2024R111)/This study was supported by Researchers Supporting Project number (RSP2024R111), King Saud University, and Riyadh, Saudi Arabia.
LinkOut - more resources
Full Text Sources
Medical

