Autophagy as a Guardian of Vascular Niche Homeostasis
- PMID: 39337582
- PMCID: PMC11432168
- DOI: 10.3390/ijms251810097
Autophagy as a Guardian of Vascular Niche Homeostasis
Abstract
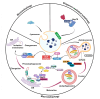
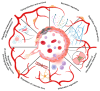
The increasing burden of vascular dysfunction on healthcare systems worldwide results in higher morbidity and mortality rates across pathologies, including cardiovascular diseases. Vasculopathy is suggested to be caused by the dysregulation of vascular niches, a microenvironment of vascular structures comprising anatomical structures, extracellular matrix components, and various cell populations. These elements work together to ensure accurate control of the vascular network. In recent years, autophagy has been recognized as a crucial regulator of the vascular microenvironment responsible for maintaining basic cell functions such as proliferation, differentiation, replicative senescence, and apoptosis. Experimental studies indicate that autophagy activation can be enhanced or inhibited in various pathologies associated with vascular dysfunction, suggesting that autophagy plays both beneficial and detrimental roles. Here, we review and assess the principles of autophagy organization and regulation in non-tumor vascular niches. Our analysis focuses on significant figures in the vascular microenvironment, highlighting the role of autophagy and summarizing evidence that supports the systemic or multiorgan nature of the autophagy effects. Finally, we discuss the critical organizational and functional aspects of the vasculogenic niche, specifically in relation to autophagy. The resulting dysregulation of the vascular microenvironment contributes to the development of vascular dysfunction.
Keywords: autophagy; vascular niche; vascular regeneration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Rouget C. Memoire Sur Les Development, La Structure et La Proprietes Physiologiques Des Capillaires Sanguines et Lymphatiques. Arch. Physiol. 1873;5:603–663.
-
- Eberth C.J. Handbuch Der Lehre von Der Gewegen Des Menschen und Der Tiereitle Bd.1. Verlag von Wilhelm Engelmann; Leipzig, Germany: 1871.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

