Prevalence of Fabry Disease in Patients on Dialysis in France
- PMID: 39337589
- PMCID: PMC11432483
- DOI: 10.3390/ijms251810104
Prevalence of Fabry Disease in Patients on Dialysis in France
Abstract
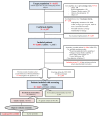
Numerous prevalence studies on Fabry disease (FD, OMIM #301500) have been conducted in dialysis populations across the world with variable and controversial results. The FABRYDIAL study aimed to estimate the prevalence of FD in patients aged 18 to 74 years on chronic dialysis in France. This cross-sectional study was conducted in patients undergoing dialysis. One hundred and twenty-four dialysis centers participated. Patients with proven causes of nephropathy unrelated to FD were excluded. Alpha-galactosidase A activity was assayed in men, and both α-galactosidase A and lyso-Gb3 were assayed in women from dried blood spots. GLA gene sequencing was performed in case of abnormal values. If a variant was identified, a diagnosis validation committee was consulted for adjudication. Among the 6032 targeted patients, 3088 were included (73.6% of the eligible patients). Biochemical results were available for 2815 (1721 men and 1094 women). A genetic variant of GLA was identified in five patients: a benign c.937G>T/p.(Asp313Tyr) variant in two individuals, a likely benign c.427G>A/(p.Ala143Thr) variant, a likely benign c.416A>G/(p.Asn139Ser) variant, and a pathogenic c.1185dupG/p.Phe396Glyfs variant. Among the screened patients, the prevalence was 0.058% [0.010;0.328] in males, 0% [0.000;0.350] in females, and 0.035% [0.006;0.201] when both genders were pooled. Among all patients aged 18-74 years undergoing dialysis without a previously known cause of nephropathy unlinked to FD, the prevalence was 0.028% [0.006;0.121]. The prevalence of FD in a cohort of French dialysis patients was low. However, considering the prognostic impact of earlier diagnosis, signs of FD should be sought in patients with nephropathies of uncertain etiology.
Keywords: Fabry disease; dialysis; genetic variants; prevalence; screening.
Conflict of interest statement
F.S., G.C. and L.J. received travel grants from Sanofi. B.K. received travel grants from Sanofi and Amicus Therapeutics. D.P.G. is a consultant for Chiesi, Idorsia, Takeda, and Sanofi. V.d.P., O.M., L.T.T.P. and T.G. declare no conflicts of interest.
Figures
References
-
- Spada M., Baron R., Elliott P.M., Falissard B., Hilz M.J., Monserrat L., Tøndel C., Tylki-Szymańska A., Wanner C., Germain D.P. The effect of enzyme replacement therapy on clinical outcomes in paediatric patients with Fabry disease—A systematic literature review by a European panel of experts. Mol. Genet. Metab. 2019;126:212–223. doi: 10.1016/j.ymgme.2018.04.007. - DOI - PubMed
-
- Terryn W., Cochat P., Froissart R., Ortiz A., Pirson Y., Poppe B., Serra A., Van Biesen W., Vanholder R., Wanner C. Fabry nephropathy: Indications for screening and guidance for diagnosis and treatment by the European Renal Best Practice. Nephrol. Dial. Transplant. 2013;28:505–517. doi: 10.1093/ndt/gfs526. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical