Chromate Affects Gene Expression and DNA Methylation in Long-Term In Vitro Experiments in A549 Cells
- PMID: 39337613
- PMCID: PMC11431867
- DOI: 10.3390/ijms251810129
Chromate Affects Gene Expression and DNA Methylation in Long-Term In Vitro Experiments in A549 Cells
Abstract
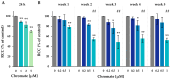
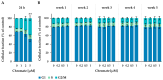
Chromate has been shown to dysregulate epigenetic mechanisms such as DNA methylation, leading to changes in gene expression and genomic instability. However, most in vitro studies are limited to short incubation periods, although chronic exposure may be more relevant for both environmental and occupational exposure. In this study, human adenocarcinoma A549 cells were treated with 1, 2 or 5 µM chromate for 24 h and compared with incubations with 0.2, 0.5 or 1 µM chromate for 1 to 5 weeks. Chromium accumulated in a pronounced time- and concentration-dependent manner after short-term treatment, whereas a plateau of intracellular chromium content was observed after long-term treatment. While short-term treatment induced a G2 arrest of the cell cycle, this effect was not observed after long-term treatment at lower concentrations. The opposite was observed for global DNA methylation: while short-term treatment showed no effect of chromate, significant dose-dependent hypomethylation was observed in the long-term experiments. Time-dependent effects were also observed in a high-throughput RT-qPCR gene expression analysis, particularly in genes related to the inflammatory response and DNA damage response. Taken together, the results suggest specific differences in toxicity profiles when comparing short-term and long-term exposure to chromate in A549 cells.
Keywords: DNA damage response; DNA methylation; chromate; epigenetic; gene expression profiles; inflammation; long-term exposure; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- International Agency for Research on Cancer (IARC) A Review of Human Carcinogens. Volume 100C. International Agency for Research on Cancer; Lyon, France: 2012. pp. 147–167. Arsenic, Metals Fibres, and Dusts.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

