Phenotypic, Metabolic, and Functional Characterization of Experimental Models of Foamy Macrophages: Toward Therapeutic Research in Atherosclerosis
- PMID: 39337629
- PMCID: PMC11432604
- DOI: 10.3390/ijms251810146
Phenotypic, Metabolic, and Functional Characterization of Experimental Models of Foamy Macrophages: Toward Therapeutic Research in Atherosclerosis
Abstract
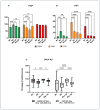
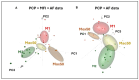
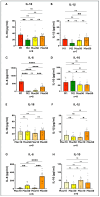
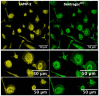
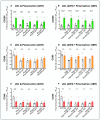
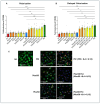
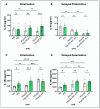
Different types of macrophages (Mφ) are involved in atherogenesis, including inflammatory Mφ and foamy Mφ (FM). Our previous study demonstrated that two-photon excited fluorescence (TPEF) imaging of NADH and FAD autofluorescence (AF) could distinguish experimental models that mimic the different atherosclerotic Mφ types. The present study assessed whether optical differences correlated with phenotypic and functional differences, potentially guiding diagnostic and therapeutic strategies. Phenotypic differences were investigated using three-dimensional principal component analysis and multi-color flow cytometry. Functional analyses focused on cytokine production, metabolic profiles, and cellular oxidative stress, in LDL dose-dependent assays, to understand the origin of AF in the FAD spectrum and assess FM ability to transition toward an immunoregulatory phenotype and function. Phenotypic studies revealed that FM models generated with acetylated LDL (Mac) were closer to immunoregulatory Mφ, while those generated with oxidized LDL (Mox) more closely resembled inflammatory Mφ. The metabolic analysis confirmed that inflammatory Mφ primarily used glycolysis, while immunoregulatory Mφ mainly depended on mitochondrial respiration. FM models employed both pathways; however, FM models generated with high doses of modified LDL showed reduced mitochondrial respiration, particularly Mox FM. Thus, the high AF in the FAD spectrum in Mox was not linked to increased mitochondrial respiration, but correlated with the dose of oxidized LDL, leading to increased production of reactive oxygen species (ROS) and lysosomal ceroid accumulation. High FAD-like AF, ROS, and ceroid accumulation were reduced by incubation with α-tocopherol. The cytokine profiles supported the phenotypic analysis, indicating that Mox FM exhibited greater inflammatory activity than Mac FM, although both could be redirected toward immunoregulatory functions, albeit to different degrees. In conclusion, in the context of immunoregulatory therapies for atherosclerosis, it is crucial to consider FM, given their prevalence in plaques and our results, as potential targets, regardless of their inflammatory status, alongside non-foamy inflammatory Mφ.
Keywords: TPEF autofluorescence; antioxidant; atherosclerosis; bioenergetic immunometabolism; cellular oxidative stress; ceroids; diagnostic and therapeutic strategies; foamy macrophages; immunoregulation; phenotype and cytokine profiling.
Conflict of interest statement
The authors declare they do not have anything to disclose regarding conflicts of interest with respect to this manuscript.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

