Temporal Dynamics of Oxidative Stress and Inflammation in Bronchopulmonary Dysplasia
- PMID: 39337630
- PMCID: PMC11431892
- DOI: 10.3390/ijms251810145
Temporal Dynamics of Oxidative Stress and Inflammation in Bronchopulmonary Dysplasia
Abstract
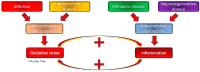
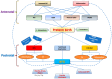
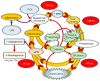
Bronchopulmonary dysplasia (BPD) is the most common lung complication of prematurity. Despite extensive research, our understanding of its pathophysiology remains limited, as reflected by the stable prevalence of BPD. Prematurity is the primary risk factor for BPD, with oxidative stress (OS) and inflammation playing significant roles and being closely linked to premature birth. Understanding the interplay and temporal relationship between OS and inflammation is crucial for developing new treatments for BPD. Animal studies suggest that OS and inflammation can exacerbate each other. Clinical trials focusing solely on antioxidants or anti-inflammatory therapies have been unsuccessful. In contrast, vitamin A and caffeine, with antioxidant and anti-inflammatory properties, have shown some efficacy, reducing BPD by about 10%. However, more than one-third of very preterm infants still suffer from BPD. New therapeutic agents are needed. A novel tripeptide, N-acetyl-lysyltyrosylcysteine amide (KYC), is a reversible myeloperoxidase inhibitor and a systems pharmacology agent. It reduces BPD severity by inhibiting MPO, enhancing antioxidative proteins, and alleviating endoplasmic reticulum stress and cellular senescence in a hyperoxia rat model. KYC represents a promising new approach to BPD treatment.
Keywords: anti-inflammatory; antioxidant; bronchopulmonary dysplasia; endoplasmic reticulum stress; inflammation; oxidative stress; senescence; temporal relationship; therapeutic intervention.
Conflict of interest statement
K.A.P.J., B.W.D. and S.N. own ReNeuroGen LLC, which is developing the drug candidate KYC as a potential therapeutic intervention in BPD. At present, KYC is in the preclinical phase of development. All other authors report no conflicts of interest.
Figures
References
-
- Stoll B.J., Hansen N.I., Bell E.F., Shankaran S., Laptook A.R., Walsh M.C., Hale E.C., Newman N.S., Schibler K., Carlo W.A., et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

