Dysbiosis in Human Urinary Microbiota May Differentiate Patients with a Bladder Cancer
- PMID: 39337643
- PMCID: PMC11432408
- DOI: 10.3390/ijms251810159
Dysbiosis in Human Urinary Microbiota May Differentiate Patients with a Bladder Cancer
Abstract
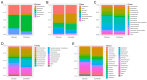
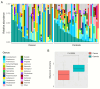
Recent interest in noninvasive diagnostic approaches has highlighted the potential of urinary microbiota as a novel biomarker for bladder cancer. This study investigated the urinary microbiota of 30 bladder cancer patients and 32 healthy controls using a specific NGS protocol that sequences eight hypervariable regions of the 16S rRNA gene, providing detailed insights into urinary microbiota composition. The relative abundance of microbial compositions in urine samples from cancer patients and healthy controls was analyzed across various taxonomic levels. No notable differences were highlighted at the phylum, class, order, and family levels. At the genus level, 53% of detected genera were represented in either cancer patients or healthy controls. Microbial diversity was significantly lower in cancer patients. The differential analysis identified five genera, Rhodanobacter, Cutibacterium, Alloscardovia, Moryella, and Anaeroglobus, that were significantly more abundant in cancer patients. Notably, Rhodanobacter was present in 20 cancer samples but absent in healthy controls. Conversely, 40 genera, including Lactobacillus, Propionibacterium, and Bifidobacterium, exhibited reduced abundance in cancer patients. These findings suggest that some genera may serve as potential biomarkers for bladder cancer, highlighting the need for further research to explore their roles in disease pathogenesis and their potential applications in diagnostics and therapeutics.
Keywords: Cutibacterium; NGS; Rhodanobacter; bladder cancer; urinary microbiota.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

