The Effects of Growth Hormone Treatment Beyond Growth Promotion in Patients with Genetic Syndromes: A Systematic Review of the Literature
- PMID: 39337654
- PMCID: PMC11432634
- DOI: 10.3390/ijms251810169
The Effects of Growth Hormone Treatment Beyond Growth Promotion in Patients with Genetic Syndromes: A Systematic Review of the Literature
Abstract
Recombinant human growth hormone therapy (rhGH) has been widely accepted as the safe treatment for short stature in children with such genetic syndromes as Prader-Willi syndrome and Turner or Noonan syndrome. Some patients with short stature and rare genetic syndromes are treated with rhGH as growth hormone-deficient individuals or as children born small for their gestational age. After years of experience with this therapy in syndromic short stature, it has been proved that there are some aspects of long-term rhGH treatment beyond growth promotion, which can justify rhGH use in these individuals. This paper summarizes the data of a literature review of the effects of rhGH treatment beyond growth promotion in selected genetic syndromes. We chose three of the most common syndromes, Prader-Willi, Turner, and Noonan, in which rhGH treatment is indicated, and three rarer syndromes, Silver-Russel, Kabuki, and Duchenne muscular dystrophy, in which rhGH treatment is not widely indicated. Many studies have shown a significant impact of rhGH therapy on body composition, resting energy expenditure, insulin sensitivity, muscle tonus, motor function, and mental and behavioral development. Growth promotion is undoubtedly the primary benefit of rhGH therapy; nevertheless, especially with genetic syndromes, the additional effects should also be considered as important indications for this treatment.
Keywords: QoL; bone; children; genetic syndromes; growth hormone treatment; metabolic effects; muscle.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
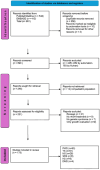
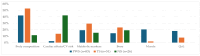
References
-
- Deal C.L., Tony M., Höybye C., Allen D.B., Tauber M., Christiansen J.S., 2011 Growth Hormone in Prader-Willi Syndrome Clinical Care Guidelines Workshop Participants Growth Hormone Research Society workshop summary: Consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2013;98:E1072–E1087. doi: 10.1210/jc.2012-3888. - DOI - PMC - PubMed
-
- Gravholt C.H., Andersen N.H., Conway G.S., Dekkers O.M., Geffner M.E., Klein K.O., Lin A.E., Mauras N., Quigley C.A., Rubin K., et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: Proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur. J. Endocrinol. 2017;177:G1–G70. doi: 10.1530/EJE-17-0430. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

