Statins in Mitigating Anticancer Treatment-Related Cardiovascular Disease
- PMID: 39337662
- PMCID: PMC11432657
- DOI: 10.3390/ijms251810177
Statins in Mitigating Anticancer Treatment-Related Cardiovascular Disease
Abstract
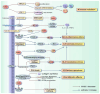
Certain anticancer therapies inevitably increase the risk of cardiovascular events, now the second leading cause of death among cancer patients. This underscores the critical need for developing effective drugs or regimens for cardiovascular protection. Statins possess properties such as antioxidative stress, anti-inflammatory effects, antifibrotic activity, endothelial protection, and immune modulation. These pathological processes are central to the cardiotoxicity associated with anticancer treatment. There is prospective clinical evidence confirming the protective role of statins in chemotherapy-induced cardiotoxicity. Numerous preclinical studies have demonstrated that statins can ameliorate heart and endothelial damage caused by radiotherapy, although clinical studies are scarce. In the animal models of trastuzumab-induced cardiomyopathy, statins provide protection through anti-inflammatory, antioxidant, and antifibrotic mechanisms. In animal and cell models, statins can mitigate inflammation, endothelial damage, and cardiac injury induced by immune checkpoint inhibitors. Chimeric antigen receptor (CAR)-T cell therapy-induced cardiotoxicity and immune effector cell-associated neurotoxicity syndrome are associated with uncontrolled inflammation and immune activation. Due to their anti-inflammatory and immunomodulatory effects, statins have been used to manage CAR-T cell therapy-induced immune effector cell-associated neurotoxicity syndrome in a clinical trial. However, direct evidence proving that statins can mitigate CAR-T cell therapy-induced cardiotoxicity is still lacking. This review summarizes the possible mechanisms of anticancer therapy-induced cardiotoxicity and the potential mechanisms by which statins may reduce related cardiac damage. We also discuss the current status of research on the protective effect of statins in anticancer treatment-related cardiovascular disease and provide directions for future research. Additionally, we propose further studies on using statins for the prevention of cardiovascular disease in anticancer treatment.
Keywords: CAR-T cell therapy; cardiotoxicity; chemotherapy; immune checkpoint inhibitor; radiotherapy; statin; targeted therapy.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

