Evidence for the Presence of Borrelia burgdorferi Biofilm in Infected Mouse Heart Tissues
- PMID: 39338441
- PMCID: PMC11434270
- DOI: 10.3390/microorganisms12091766
Evidence for the Presence of Borrelia burgdorferi Biofilm in Infected Mouse Heart Tissues
Abstract
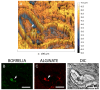
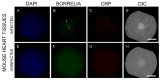
Borrelia burgdorferi, the bacterium responsible for Lyme disease, has been shown to form antimicrobial-tolerant biofilms, which protect it from unfavorable conditions. Bacterial biofilms are known to significantly contribute to severe inflammation, such as carditis, a common manifestation of Lyme disease. However, the role of B. burgdorferi biofilms in the development of Lyme carditis has not been thoroughly investigated due to the absence of an appropriate model system. In this study, we examined heart tissues from mice infected with B. burgdorferi for the presence of biofilms and inflammatory markers using immunohistochemistry (IHC), combined fluorescence in situ hybridization FISH/IHC, 3D microscopy, and atomic force microscopy techniques. Our results reveal that B. burgdorferi spirochetes form aggregates with a known biofilm marker (alginate) in mouse heart tissues. Furthermore, these biofilms induce inflammation, as indicated by elevated levels of murine C-reactive protein near the biofilms. This research provides evidence that B. burgdorferi can form biofilms in mouse heart tissue and trigger inflammatory processes, suggesting that the mouse model is a valuable tool for future studies on B. burgdorferi biofilms.
Keywords: C-reactive protein; atomic force microscopy; biofilm; carditis; immunohistochemistry; inflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Liegner K.B., Shapiro J.R., Ramsay D., Halperin A.J., Hogrefe W., Kong L. Recurrent erythema migrans despite extended antibiotic treatment with minocycline in a patient with persisting Borrelia burgdorferi infection. J. Am. Acad. Dermatol. 1993;28:312–314. doi: 10.1016/0190-9622(93)70043-S. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

