Chikungunya Virus RNA Secondary Structures Impact Defective Viral Genome Production
- PMID: 39338469
- PMCID: PMC11434300
- DOI: 10.3390/microorganisms12091794
Chikungunya Virus RNA Secondary Structures Impact Defective Viral Genome Production
Abstract
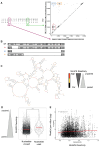
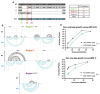
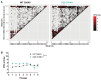
Chikungunya virus (CHIKV) is a mosquito-borne RNA virus that poses an emerging threat to humans. In a manner similar to other RNA viruses, CHIKV encodes an error-prone RNA polymerase which, in addition to producing full-length genomes, gives rise to truncated, non-functional genomes, which have been coined defective viral genomes (DVGs). DVGs have been intensively studied in the context of therapy, as they can inhibit viral replication and dissemination in their hosts. In this work, we interrogate the influence of viral RNA secondary structures on the production of CHIKV DVGs. We experimentally map RNA secondary structures of the CHIKV genome using selective 2'-hydroxyl acylation analyzed by primer extension and mutational profiling (SHAPE-MaP), which couples chemical labelling with next-generation sequencing. We correlate the inferred secondary structure with preferred deletion sites of CHIKV DVGs. We document an increased probability of DVG generation with truncations at unpaired nucleotides within the secondary structure. We then generated a CHIKV mutant bearing synonymous changes at the nucleotide level to disrupt the existing RNA secondary structure (CHIKV-D2S). We show that CHIKV-D2S presents altered DVG generation compared to wild-type virus, correlating with the change in RNA secondary structure obtained by SHAPE-MaP. Our work thus demonstrates that RNA secondary structure impacts CHIKV DVG production during replication.
Keywords: RNA secondary structure; SHAPE-MaP; chikungunya virus; defective viral genome.
Conflict of interest statement
L.I.L., V.B, T.V. and M.V. are co-inventors of the DI 2018-21 patent. The rest of the authors declare no conflicts of interest. None of the funders described below had any influence on study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures

References
-
- Bouquillard E., Fianu A., Bangil M., Charlette N., Ribéra A., Michault A., Favier F., Simon F., Flipo R.-M. Rheumatic Manifestations Associated with Chikungunya Virus Infection: A Study of 307 Patients with 32-Month Follow-up (RHUMATOCHIK Study) Jt. Bone Spine Rev. Rhum. 2018;85:207–210. doi: 10.1016/j.jbspin.2017.01.014. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

