Sulfate-Reducing Bacteria Induce Pro-Inflammatory TNF-α and iNOS via PI3K/Akt Pathway in a TLR 2-Dependent Manner
- PMID: 39338507
- PMCID: PMC11434237
- DOI: 10.3390/microorganisms12091833
Sulfate-Reducing Bacteria Induce Pro-Inflammatory TNF-α and iNOS via PI3K/Akt Pathway in a TLR 2-Dependent Manner
Abstract
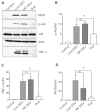
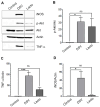
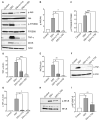
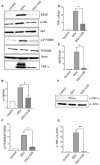
Desulfovibrio, resident gut sulfate-reducing bacteria (SRB), are found to overgrow in diseases such as inflammatory bowel disease and Parkinson's disease. They activate a pro-inflammatory response, suggesting that Desulfovibrio may play a causal role in inflammation. Class I phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway regulates key events in the inflammatory response to infection. Dysfunctional PI3K/Akt signaling is linked to numerous diseases. Bacterial-induced PI3K/Akt pathway may be activated downstream of toll-like receptor (TLR) signaling. Here, we tested the hypothesis that Desulfovibrio vulgaris (DSV) may induce tumor necrosis factor alpha (TNF-α) and inducible nitric oxide synthase (iNOS) expression via PI3K/Akt in a TLR 2-dependent manner. RAW 264.7 macrophages were infected with DSV, and protein expression of p-Akt, p-p70S6K, p-NF-κB, p-IkB, TNF-α, and iNOS was measured. We found that DSV induced these proteins in a time-dependent manner. Heat-killed and live DSV, but not bacterial culture supernatant or a probiotic Lactobacillus plantarum, significantly caused PI3K/AKT/TNF/iNOS activation. LY294002, a PI3K/Akt signaling inhibitor, and TL2-C29, a TLR 2 antagonist, inhibited DSV-induced PI3K/AKT pathway. Thus, DSV induces pro-inflammatory TNF-α and iNOS via PI3K/Akt pathway in a TLR 2-dependent manner. Taken together, our study identifies a novel mechanism by which SRB such as Desulfovibrio may trigger inflammation in diseases associated with SRB overgrowth.
Keywords: Desulfovibrio vulgaris (DSV); inducible nitric oxide synthase (iNOS); phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) (PI3K/AKT); toll-like receptor 2 (TLR 2); tumor necrosis factor-α (TNF-α).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Dzierzewicz Z., Szczerba J., Lodowska J., Wolny D., Gruchlik A., Orchel A., Weglarz L. The role of Desulfovibrio desulfuricans lipopolysaccharides in modulation of periodontal inflammation through stimulation of human gingival fibroblasts. Arch. Oral Biol. 2010;55:515–522. doi: 10.1016/j.archoralbio.2010.05.001. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

