Immunological Considerations for the Development of an Effective Herpes Vaccine
- PMID: 39338520
- PMCID: PMC11434158
- DOI: 10.3390/microorganisms12091846
Immunological Considerations for the Development of an Effective Herpes Vaccine
Abstract
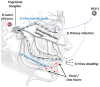
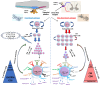
Research is underway to develop a vaccine to prevent and cure infection from herpes simplex virus (HSV). It emphasizes the critical need for immunization to address public health issues and the shortcomings of existing treatment options. Furthermore, studies on the HSV vaccine advance the field of immunology and vaccine creation, which may help in the battle against other viral illnesses. The current lack of such a vaccine is, in part, due to herpes viral latency in sensory ganglions. Current vaccines rely on tissue-resident memory CD8+ T cells, which are known to provide protection against subsequent HSV reinfection and reactivation without correlating with other immune subsets. For that reason, there is no effective vaccine that can provide protection against latent or recurrent herpes infection. This review focuses on conventional methods for evaluating the efficacy of a herpes vaccine using differential CD8+ T cells and important unaccounted immune aspects for designing an effective vaccine against herpes.
Keywords: CD8 T cells; adaptive immunity; herpes simplex virus (HSV); innate immunity; memory T cells; vaccine design; virus latency.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Therapeutic immunization with a mixture of herpes simplex virus 1 glycoprotein D-derived “asymptomatic” human CD8+ T-cell epitopes decreases spontaneous ocular shedding in latently infected HLA transgenic rabbits: association with low frequency of local PD-1+ TIM-3+ CD8+ exhausted T cells.J Virol. 2015 Jul;89(13):6619-32. doi: 10.1128/JVI.00788-15. J Virol. 2015. PMID: 25878105 Free PMC article.
-
CXCL10/CXCR3-Dependent Mobilization of Herpes Simplex Virus-Specific CD8+ TEM and CD8+ TRM Cells within Infected Tissues Allows Efficient Protection against Recurrent Herpesvirus Infection and Disease.J Virol. 2017 Jun 26;91(14):e00278-17. doi: 10.1128/JVI.00278-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28468883 Free PMC article.
-
Therapeutic Mucosal Vaccination of Herpes Simplex Virus 2-Infected Guinea Pigs with Ribonucleotide Reductase 2 (RR2) Protein Boosts Antiviral Neutralizing Antibodies and Local Tissue-Resident CD4+ and CD8+ TRM Cells Associated with Protection against Recurrent Genital Herpes.J Virol. 2019 Apr 17;93(9):e02309-18. doi: 10.1128/JVI.02309-18. Print 2019 May 1. J Virol. 2019. PMID: 30787156 Free PMC article.
-
Combinatorial Herpes Simplex Vaccine Strategies: From Bedside to Bench and Back.Front Immunol. 2022 Apr 25;13:849515. doi: 10.3389/fimmu.2022.849515. eCollection 2022. Front Immunol. 2022. PMID: 35547736 Free PMC article. Review.
-
Vaccines for Herpes Simplex: Recent Progress Driven by Viral and Adjuvant Immunology.Methods Mol Biol. 2020;2060:31-56. doi: 10.1007/978-1-4939-9814-2_2. Methods Mol Biol. 2020. PMID: 31617171 Review.
Cited by
-
Salmonella-Based Vaccine: A Promising Strategy for Type 1 Diabetes.Vaccines (Basel). 2025 Apr 14;13(4):405. doi: 10.3390/vaccines13040405. Vaccines (Basel). 2025. PMID: 40333284 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

