Modifications of Mitochondrial Network Morphology Affect the MAVS-Dependent Immune Response in L929 Murine Fibroblasts during Ectromelia Virus Infection
- PMID: 39338909
- PMCID: PMC11434706
- DOI: 10.3390/pathogens13090717
Modifications of Mitochondrial Network Morphology Affect the MAVS-Dependent Immune Response in L929 Murine Fibroblasts during Ectromelia Virus Infection
Abstract
Since smallpox vaccination was discontinued in 1980, there has been a resurgence of poxvirus infections, particularly the monkeypox virus. Without a global recommendation to use the smallpox vaccine, the population is not immune, posing a severe threat to public health. Given these circumstances, it is crucial to understand the relationship between poxviruses and their hosts. Therefore, this study focuses on the ectromelia virus, the causative agent of mousepox, which serves as an excellent model for studying poxvirus pathogenesis. Additionally, we investigated the role of mitochondria in innate antiviral immunity during ECTV infection, focusing specifically on mitochondrial antiviral signaling protein. The study used a Moscow strain of ECTV and L929 mouse fibroblasts. Cells were treated with ECTV and chemical modulators of mitochondrial network: Mdivi-1 and CCCP. Our investigation revealed that an elongated mitochondrial network attenuates the suppression of MAVS-dependent immunity by ECTV and reduces ECTV replication in L929 fibroblasts compared to cells with an unaltered mitochondrial network. Conversely, a fragmented mitochondrial network reduces the number of progeny virions while increasing the inhibition of the virus-induced immune response during infection. In conclusion, our study showed that modifications of mitochondrial network morphology alter MAVS-dependent immunity in ECTV-infected mouse L929 fibroblasts.
Keywords: ECTV; MAVS; immunity; mitochondrial dynamics; mitochondrial network; poxviruses.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




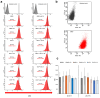





References
-
- Past U.S. Cases and Outbreaks|Mpox|Poxvirus|CDC Dostępne na. [(accessed on 24 February 2024)]; Available online: https://www.cdc.gov/poxvirus/mpox/outbreak/us-outbreaks.html.
-
- Biernacka Z., Gregorczyk-Zboroch K., Lasocka I., Ostrowska A., Struzik J., Gieryńska M., Toka F.N., Szulc-Dąbrowska L. Ectromelia Virus Affects the Formation and Spatial Organization of Adhesive Structures in Murine Dendritic Cells In Vitro. Int. J. Mol. Sci. 2023;25:558. doi: 10.3390/ijms25010558. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

