Comprehensive Analysis of Cetuximab Critical Quality Attributes: Impact of Handling on Antigen-Antibody Binding
- PMID: 39339258
- PMCID: PMC11435379
- DOI: 10.3390/pharmaceutics16091222
Comprehensive Analysis of Cetuximab Critical Quality Attributes: Impact of Handling on Antigen-Antibody Binding
Abstract
Background/objectives: Cetuximab, formulated in Erbitux® (5 mg/mL), is a therapeutic monoclonal antibody (mAb) widely used in several cancer treatments. Currently, there is insufficient knowledge about the behavior of cetuximab with regard to the risk associated with its routine handling or unintentional mishandling in hospitals. Forced degradation studies can simulate these conditions and provide insights into the biophysical and biochemical properties of mAbs.
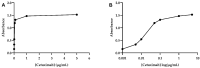
Methods: In this study, we conducted a deep physicochemical and functional characterization of the critical quality attributes of cetuximab in control samples and under controlled degraded conditions, including freeze-thaw cycles, heat, agitation, and light exposure. To achieve this purpose, we used a set of proper analytical techniques, including CD, IT-FS, DLS, SE/UHPLC-UV, UHPLC-MS/MS, and ELISA, to check functionality based on antigen-antibody binding.
Results: The results revealed that light exposure was the stress stimuli with the greatest impact on the drug product, leading to the formation of non-natural oligomers, fragmentation, and oxidation of methionine residues. Additionally, cetuximab (Erbitux®, 5 mg/mL) showed a tendency to aggregate when submitted to 60 °C for 1 h. In terms of functionality, cetuximab (Erbitux®, 5 mg/mL) samples were found to be affected when subjected to freeze-thaw cycles, 60 °C (1 h), and when exposed to light (daylight with room temperature excursion and accelerated light exposure).
Conclusions: Thus, we suggest that Erbitux® (5 mg/mL) should be shielded from these environmental conditions, as they compromise both the safety and efficacy of the drug product.
Keywords: ELISA; Erbitux® analysis; cetuximab characterization; comprehensive analysis; forced degradation; peptide mapping-RP/UHPLC-MS/MS; stability study.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- European Medicines Agency (EMA) Erbitux European Public Assessment Report. Product Information 2022. [(accessed on 10 March 2024)]. Available online: https://www.ema.europa.eu/en/documents/product-information/erbitux-epar-....
-
- Galizia G., Lieto E., De Vita F., Orditura M., Castellano P., Troiani T., Imperatore V., Ciardiello F. Cetuximab, a Chimeric Human Mouse Anti-Epidermal Growth Factor Receptor Monoclonal Antibody, in the Treatment of Human Colorectal Cancer. Oncogene. 2007;26:3654–3660. doi: 10.1038/sj.onc.1210381. - DOI - PubMed
-
- Suárez I., Salmerón-García A., Cabeza J., Capitán-Vallvey L.F., Navas N. Development and Use of Specific ELISA Methods for Quantifying the Biological Activity of Bevacizumab, Cetuximab and Trastuzumab in Stability Studies. J. Chromatogr. B. 2016;1032:155–164. doi: 10.1016/j.jchromb.2016.05.045. - DOI - PubMed
-
- Souza A.L.R.d., Amorim A.C.F., Cintra E.R., Ferreira N.N., Silva L.A.D., Hayasaki T.G., Diniz D.G.A., Lima E.M. Development and Validation of a Rapid RP-HPLC Method for Simultaneous Quantification of Paclitaxel and Cetuximab in Immunoliposomes. Talanta. 2021;225:121988. doi: 10.1016/j.talanta.2020.121988. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

