Computational Exploration of the Mechanism of Action of a Sorafenib-Containing Ruthenium Complex as an Anticancer Agent for Photoactivated Chemotherapy
- PMID: 39339293
- PMCID: PMC11433670
- DOI: 10.3390/molecules29184298
Computational Exploration of the Mechanism of Action of a Sorafenib-Containing Ruthenium Complex as an Anticancer Agent for Photoactivated Chemotherapy
Abstract
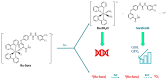
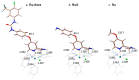
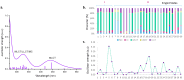
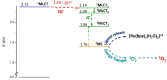
Ruthenium(II) polypyridyl complexes are being tested as potential anticancer agents in different therapies, which include conventional chemotherapy and light-activated approaches. A mechanistic study on a recently synthesized dual-action Ru(II) complex [Ru(bpy)2(sora)Cl]+ is described here. It is characterized by two mono-dentate leaving ligands, namely, chloride and sorafenib ligands, which make it possible to form a di-aquo complex able to bind DNA. At the same time, while the released sorafenib can induce ferroptosis, the complex is also able to act as a photosensitizer according to type II photodynamic therapy processes, thus generating one of the most harmful cytotoxic species, 1O2. In order to clarify the mechanism of action of the drug, computational strategies based on density functional theory are exploited. The photophysical properties of the complex, which include the absorption spectrum, the kinetics of ISC, and the character of all the excited states potentially involved in 1O2 generation, as well as the pathway providing the di-aquo complex, are fully explored. Interestingly, the outcomes show that light is needed to form the mono-aquo complex, after releasing both chloride and sorafenib ligands, while the second solvent molecule enters the coordination sphere of the metal once the system has come back to the ground-state potential energy surface. In order to simulate the interaction with canonical DNA, the di-aquo complex interaction with a guanine nucleobase as a model has also been studied. The whole study aims to elucidate the intricate details of the photodissociation process, which could help with designing tailored metal complexes as potential anticancer agents.
Keywords: ISC; PACT; PDT; photosensitizer; ruthenium complexes; sorafenib.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

