Bioassay-Guided Isolation and Identification of Xanthine Oxidase Inhibitory Constituents from the Fruits of Chaenomeles speciosa (Sweet) Nakai
- PMID: 39339463
- PMCID: PMC11434067
- DOI: 10.3390/molecules29184468
Bioassay-Guided Isolation and Identification of Xanthine Oxidase Inhibitory Constituents from the Fruits of Chaenomeles speciosa (Sweet) Nakai
Abstract
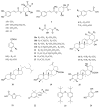
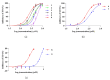
Chaenomeles speciosa (Sweet) Nakai (C. speciosa) is a traditional Chinese herbal medicine that possesses not only abundant nutritional value but also significant medicinal properties. The extracts of C. speciosa fruits effectively reduce urate levels, but the specific chemical constituents responsible for this effect in C. speciosa fruits are still unknown. Therefore, this study aims to investigate and analyze the structure-activity relationships of these constituents to better understand their ability to lower uric acid. Activity-guided fractionation and purification processes were used to isolate compounds with xanthine oxidase (XO) inhibitory activity from C. speciosa fruits, resulting in three extracts: petroleum ether, ethyl acetate, and n-butanol. The ethyl acetate and n-butanol fractions showed strong activity and underwent further separation and purification using chromatographic techniques. Twenty-four compounds were isolated and identified, with nine showing potent activity, including chlorogenic acid, methyl chlorogenate, butyl chlorogenate, ethyl chlorogenate, cryptochlorogenic acid methyl ester, caffeic acid, p-coumaric acid, benzoic acid and protocatechuic acid. The docking analysis showed that these compounds interacted with amino acid residues in the active site of XO through hydrogen bonding and hydrophobic interactions. These findings suggest that these compounds help reduce uric acid in C. speciosa, supporting further investigation into their mechanism of action.
Keywords: Chaenomeles speciosa (Sweet) Nakai fruits; bioassay-guided; chemical composition; molecular docking; uric acid; xanthine oxidase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Cao W., Fang Y., Wu T., Liang F., Cheng Y., Salah M., Pan S., Xu X. Insights from multispectral and molecular docking investigation on the xanthine oxidase inhibition by 1,4-dicaffeoylquinic acid. J. Mol. Struct. 2020;1219:128475. doi: 10.1016/j.molstruc.2020.128475. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

