Zn/Cr-MOFs/TiO2 Composites as Adsorbents for Levofloxacin Hydrochloride Removal
- PMID: 39339472
- PMCID: PMC11434544
- DOI: 10.3390/molecules29184477
Zn/Cr-MOFs/TiO2 Composites as Adsorbents for Levofloxacin Hydrochloride Removal
Abstract
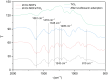
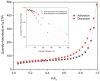
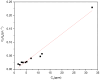
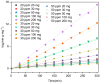
The Zn/Cr-MOFs/TiO2 composites were synthesized using the solvothermal method. XRD, FTIR, and SEM techniques were utilized to characterize the Zn/Cr-MOFs/TiO2 composites employed for simulating levofloxacin hydrochloride in wastewater. The impact of the mass of the Zn/Cr-MOFs/TiO2 composite, concentration of levofloxacin hydrochloride, solution pH, and temperature on the adsorption performance was investigated. Experimental findings indicated that at pH 6, the maximum removal efficiency of levofloxacin hydrochloride by the Zn/Cr-MOFs/TiO2 composite was achieved at 88.8%, with an adsorption capacity of 246.3 mg/g. To analyze the experimental data, both pseudo-first-order and pseudo-second-order kinetics models were applied, revealing that the pseudo-second-order model provided a better fit to the data. Additionally, Langmuir and Freundlich isotherm models were used to study equilibrium adsorption behavior and showed good agreement with both kinetic modeling and Langmuir isotherm analysis results. These observations suggest that monolayer adsorption predominates during the removal process of levofloxacin hydrochloride by Zn/Cr-MOFs/TiO2 composites.
Keywords: MOFs; adsorption; antibiotic; composites.
Conflict of interest statement
The authors declare no competing financial interests.
Figures









References
-
- Chen Y.-Z., Zhang R., Jiao L., Jiang H.-L. Metal-organic framework derived porous materials for catalysis. Coord. Chem. Rev. 2018;362:1–23. doi: 10.1016/j.ccr.2018.02.008. - DOI
-
- Sheng W., Shi J.-L., Hao H., Li X., Lang X. Polyimide-TiO2 Hybrid Photocatalysis: Visible Light-Promoted Selective Aerobic Oxidation of Amines. Chem. Eng. J. 2020;379:122399. doi: 10.1016/j.cej.2019.122399. - DOI
LinkOut - more resources
Full Text Sources

