Temperature Dependence and the Effects of Ultraviolet Radiation on the Ultrastructure and Photosynthetic Activity of Carpospores in Sub-Antarctic Red Alga Iridaea cordata (Turner) Bory 1826
- PMID: 39339522
- PMCID: PMC11435075
- DOI: 10.3390/plants13182547
Temperature Dependence and the Effects of Ultraviolet Radiation on the Ultrastructure and Photosynthetic Activity of Carpospores in Sub-Antarctic Red Alga Iridaea cordata (Turner) Bory 1826
Abstract
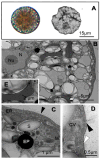
The short-term effects of UV radiation and low temperature on ultrastructure, photosynthetic activity (measured as the maximal photochemical quantum yield of photosystem II: Fv/Fm), chlorophyll-a (Chl-a) contents, and UV-absorbing compounds on the carpospores of Iridaea cordata from a sub-Antarctic population were investigated. Exposure to both photosynthetically active radiation (PAR) and PAR + UV for 4 h caused ultrastructural modifications in all treatments. Under PAR + UV at 2 °C, a disruption of the chloroplast's internal organization was observed. Plastoglobuli were often found in carpospores exposed to 2 °C. 'Electron dense particles', resembling physodes of brown algae, were detected for the first time in cells exposed to PAR and PAR + UV at 8 °C. Fv/Fm decreased following 4 h exposure at 2 °C under PAR + UV (64%) and PAR (25%). At 8 °C, Fv/Fm declined by 21% only under PAR + UV. The photosynthesis of carpospores previously treated with UV partially recovered after a 4 h exposure under dim light. UV-absorbing compounds were degraded in all radiation and temperature treatments without recovery after a 4 h dim light period. Chl-a did not change, whereas total carotenoids increased under PAR at 8 °C The study indicates that although carpospores of I. cordata exhibit photoprotective mechanisms, UV radiation strongly damages their ultrastructure and physiology, which were exacerbated under low temperatures.
Keywords: photochemistry; propagules; red algae; stress tolerance; sub-Antarctic region; ultrastructure.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Amsler C., Reed D., Neushul M. The microclimate inhabited by macroalgal propagules. Eur. J. Phycol. 1992;27:253–270. doi: 10.1080/00071619200650251. - DOI
-
- Zacher K. The susceptibility of spores and propagules of Antarctic seaweeds to UV and photosynthetically active radiation—Field versus laboratory experiments. J. Exp. Mar. Biol. Ecol. 2014;458:57–63. doi: 10.1016/j.jembe.2014.05.007. - DOI
-
- Coelho S.M., Rijstenbil J.W., Brown M.T. Impacts of anthropogenic stresses on the early development stages of seaweeds. J. Aquat. Ecosyst. Stress Recov. 2000;7:317–333. doi: 10.1023/A:1009916129009. - DOI
-
- Véliz K., Edding M., Tala F., Gómez I. Effects of ultraviolet radiation on different life cycle stages of the south Pacific kelps, Lessonia nigrescens and Lessonia trabeculata (Laminariales, Phaeophyceae) Mar. Biol. 2006;149:1015–1024. doi: 10.1007/s00227-006-0301-9. - DOI
-
- Wiencke C., Roleda M.Y., Gruber A., Clayton M.N., Bischof K. Susceptibility of zoospores to UV radiation determines upper depth distribution limit of Arctic kelps: Evidence through field experiments. J. Ecol. 2006;94:455–463. doi: 10.1111/j.1365-2745.2006.01102.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

