Biomarkers for Health Functional Foods in Metabolic Dysfunction-Associated Steatotic Liver Disorder (MASLD) Prevention: An Integrative Analysis of Network Pharmacology, Gut Microbiota, and Multi-Omics
- PMID: 39339660
- PMCID: PMC11434757
- DOI: 10.3390/nu16183061
Biomarkers for Health Functional Foods in Metabolic Dysfunction-Associated Steatotic Liver Disorder (MASLD) Prevention: An Integrative Analysis of Network Pharmacology, Gut Microbiota, and Multi-Omics
Abstract
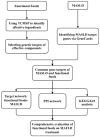
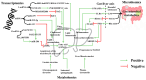
Metabolic dysfunction-associated steatotic liver disorder (MASLD) is increasingly prevalent globally, highlighting the need for preventive strategies and early interventions. This comprehensive review explores the potential of health functional foods (HFFs) to maintain healthy liver function and prevent MASLD through an integrative analysis of network pharmacology, gut microbiota, and multi-omics approaches. We first examined the biomarkers associated with MASLD, emphasizing the complex interplay of genetic, environmental, and lifestyle factors. We then applied network pharmacology to identify food components with potential beneficial effects on liver health and metabolic function, elucidating their action mechanisms. This review identifies and evaluates strategies for halting or reversing the development of steatotic liver disease in the early stages, as well as biomarkers that can evaluate the success or failure of such strategies. The crucial role of the gut microbiota and its metabolites for MASLD prevention and metabolic homeostasis is discussed. We also cover state-of-the-art omics approaches, including transcriptomics, metabolomics, and integrated multi-omics analyses, in research on preventing MASLD. These advanced technologies provide deeper insights into physiological mechanisms and potential biomarkers for HFF development. The review concludes by proposing an integrated approach for developing HFFs targeting MASLD prevention, considering the Korean regulatory framework. We outline future research directions that bridge the gap between basic science and practical applications in health functional food development. This narrative review provides a foundation for researchers and food industry professionals interested in developing HFFs to support liver health. Emphasis is placed on maintaining metabolic balance and focusing on prevention and early-stage intervention strategies.
Keywords: HFFs; gut microbiota; metabolic dysfunction-associated steatotic liver disease; omics approach.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Eslam M., Sarin S.K., Wong V.W.-S., Fan J.-G., Kawaguchi T., Ahn S.H., Zheng M.-H., Shiha G., Yilmaz Y., Gani R., et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 2020;14:889–919. doi: 10.1007/s12072-020-10094-2. - DOI - PubMed
-
- Dong X., Li J.-M., Lu X.-L., Lin X.-Y., Hong M.-Z., Weng S., Pan J.-S. Global burden of adult non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) has been steadily increasing over the past decades and is expected to persist in the future. Transl. Gastroenterol. Hepatol. 2024;9:33. doi: 10.21037/tgh-23-118. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

