Progress in the Study of Animal Models of Metabolic Dysfunction-Associated Steatotic Liver Disease
- PMID: 39339720
- PMCID: PMC11435380
- DOI: 10.3390/nu16183120
Progress in the Study of Animal Models of Metabolic Dysfunction-Associated Steatotic Liver Disease
Abstract
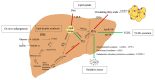
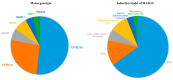
Metabolic dysfunction-associated steatotic liver disease (MASLD) has recently been proposed as an alternative term to NAFLD. MASLD is a globally recognized chronic liver disease that poses significant health concerns and is frequently associated with obesity, insulin resistance, and hyperlipidemia. To better understand its pathogenesis and to develop effective treatments, it is essential to establish suitable animal models. Therefore, attempts have been made to establish modelling approaches that are highly similar to human diet, physiology, and pathology to better replicate disease progression. Here, we reviewed the pathogenesis of MASLD disease and summarised the used animal models of MASLD in the last 7 years through the PubMed database. In addition, we have summarised the commonly used animal models of MASLD and describe the advantages and disadvantages of various models of MASLD induction, including genetic models, diet, and chemically induced models, to provide directions for research on the pathogenesis and treatment of MASLD.
Keywords: MASLD; animal model; pathology; physiology.
Conflict of interest statement
The authors declare no conflicts of interest regarding the publication of this article.
Figures


References
-
- Rinella M.E., Lazarus J.V., Ratziu V., Francque S.M., Sanyal A.J., Kanwal F., Romero D., Abdelmalek M.F., Anstee Q.M., Arab J.P., et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966–1986. doi: 10.1097/hep.0000000000000520. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

