An Oral Botanical Supplement Improves Small Intestinal Bacterial Overgrowth (SIBO) and Facial Redness: Results of an Open-Label Clinical Study
- PMID: 39339748
- PMCID: PMC11435404
- DOI: 10.3390/nu16183149
An Oral Botanical Supplement Improves Small Intestinal Bacterial Overgrowth (SIBO) and Facial Redness: Results of an Open-Label Clinical Study
Abstract
Background: Small intestinal bacterial overgrowth (SIBO) is a common, yet underdiagnosed, gut condition caused by gut dysbiosis. A previous study has shown the potential of herbal therapy, providing equivalent results to rifaximin.
Objectives: The objective of this study was to assess how the use of an oral botanical regimen may modulate the gut microbiome, facial erythema, and intestinal permeability in those with SIBO.
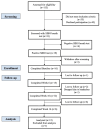
Methods: This was an open-label prospective study of adults that had lactulose breath test-confirmed SIBO. Participants received a 10-week oral supplementation of a Biocidin liquid tincture and GI Detox+. If participants were found to be non-responsive to treatment after 10 weeks with a persistently positive lactulose breath test, a third oral supplement, Olivirex, was administered for an additional 4 weeks. Lactulose breath tests were administered at baseline, weeks 6, 10, and 14 to assess for SIBO status. A high-resolution photographic analysis system was utilized to analyze changes in facial erythema. Stool sample collections and venipuncture were performed to analyze the gut microbiome and intestinal permeability.
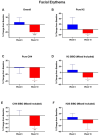
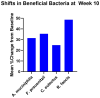
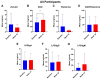
Results: A total of 33 subjects were screened with breath testing, and 19 subjects were found to have SIBO. Three of the subjects withdrew during the screening period prior to baseline, and sixteen subjects enrolled. Four subjects dropped out after baseline. Hydrogen-dominant SIBO was the most common subtype of SIBO, followed by methane and hydrogen sulfide. The botanical regimen was most effective for hydrogen- and hydrogen sulfide-dominant SIBO, leading to negative breath test results at week 10 in 42.8% and 66.7% of participants, respectively. Compared to baseline, supplementation with the botanical regimen led to positive shifts in short-chain fatty acid-producing bacteria such as A. muciniphila, F. prausnitzii, C. eutectus, and R. faecis by 31.4%, 35.4%, 24.8%, and 48.7% percent at week 10, respectively. The mean abundance of Firmicutes decreased by 20.2%, Bacteroides increased by 30%, and the F/B ratio decreased by 25.4% at week 10 compared to baseline. At week 10, there was a trending 116% increase in plasma LPS/IgG (p = 0.08). There were no significant changes in plasma zonulin, DAO, histamine, DAO/histamine, LPS/IgG, LPS/IgA, or LPS/IgM. Facial erythema was not statistically different at week 6, but at week 10, there was a 20% decrease (p = 0.001) in redness intensity. Among the patients that extended to week 14, there was no statistical change in erythema.
Conclusions: Supplementation with an antimicrobial botanical supplemental regimen may have therapeutic potential in hydrogen and hydrogen-sulfide subtypes of SIBO. Furthermore, the botanical supplemental regimen may reduce facial erythema, increase SCFA-producing bacteria, decrease the F/B ratio, and modulate markers of intestinal permeability.
Keywords: SIBO; botanical; facial erythema; gut microbiome; gut–skin axis; rosacea.
Conflict of interest statement
RKS serves as a scientific advisor for LearnHealth, Arbonne, and Codex Labs and has served as a consultant or speaker for Burt’s Bees, Novozymes, Almirall, Novartis, Sanofi, Bristol Myers Squibb, Pfizer, Nutrafol, Galderma, Abbvie, Leo, UCB, Sun, and Regeneron Pharmaceuticals. All other authors declare no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

