Transplant-Acquired Food Allergy in Children
- PMID: 39339801
- PMCID: PMC11434934
- DOI: 10.3390/nu16183201
Transplant-Acquired Food Allergy in Children
Abstract
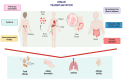
Background: Organ transplantation in children is a vital procedure for those with end-stage organ failure, but it has been linked to the development of post-transplant allergies, especially food allergies. This phenomenon, known as transplant-acquired food allergy (TAFA), is becoming increasingly recognized, though its mechanisms remain under investigation. Pediatric transplant recipients often require lifelong immunosuppressive therapy to prevent graft rejection, which can alter immune function and heighten the risk of allergic reactions. Our review aimed to gather the latest evidence on TAFA.
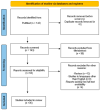
Methods: We conducted a PubMed search from 25 June to 5 July 2024, using specific search terms, identifying 143 articles. After screening, 36 studies were included: 24 retrospective studies, 1 prospective study, 2 cross-sectional researches, and 9 case reports/series.
Results: Most studies focused on liver transplants in children. The prevalence of food allergies ranged from 3.3% to 54.3%. Tacrolimus, alongside corticosteroids, was the most commonly used immunosuppressive therapy. In addition to food allergies, some patients developed atopic dermatitis, asthma, and rhinitis. Allergic symptoms typically emerged within a year post-transplant, with common allergens including milk, eggs, fish, nuts, soy, wheat, and shellfish. Both IgE-mediated and non-IgE-mediated reactions were observed, with treatment often involving the removal of offending foods and the use of adrenaline when necessary.
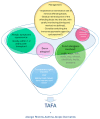
Conclusions: Consistent immunological monitoring, such as skin prick tests and IgE level assessments, is essential for early detection and management of allergies in these patients. Understanding the link between transplantation and allergy development is crucial for improving long-term outcomes for pediatric transplant recipients.
Keywords: cyclosporine; food allergies; food allergies post-transplant; food hypersensitivity; graft rejection; immunosuppressive agents; intestinal permeability; organ transplantation; pediatric transplantation; tacrolimus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

