Limosilactobacillus reuteri Alleviates Anxiety-like Behavior and Intestinal Symptoms in Two Stressed Mouse Models
- PMID: 39339809
- PMCID: PMC11434693
- DOI: 10.3390/nu16183209
Limosilactobacillus reuteri Alleviates Anxiety-like Behavior and Intestinal Symptoms in Two Stressed Mouse Models
Abstract
Background/objectives: Limosilactobacillus (Lm.) reuteri is a widely utilized probiotic, recognized for its significant role in alleviating symptoms associated with gastrointestinal and psychiatric disorders. However, the effectiveness of Lm. reuteri is strain-specific, and its genetic diversity leads to significant differences in phenotypes among different strains. This study aims to identify potential probiotic strains by comparing the strain-specific characteristics of Lm. reuteri to better understand their efficacy and mechanisms in alleviating stress-induced anxiety-like behaviors and gastrointestinal symptoms.
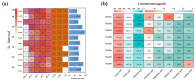
Methods: We cultivated 11 strains of Lm. reuteri from healthy human samples and conducted phenotypic and genomic characterizations. Two strains, WLR01 (=GOLDGUT-LR99) and WLR06, were screened as potential probiotics and were tested for their efficacy in alleviating anxiety-like behavior and intestinal symptoms in mouse models subjected to sleep deprivation (SD) and water avoidance stress (WAS).
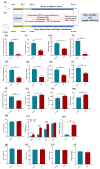
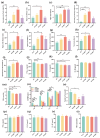
Results: The results showed that the selected strains effectively improved mouse behaviors, including cognitive impairment and inflammatory response, as well as improving anxiety and regulating gut microbiota composition. The improvements with WLR01 were associated with the regulation of the NLRP3 inflammasome pathway in the SD model mice and were associated with visceral hypersensitivity and intestinal integrity in the WAS model mice.
Conclusions: In summary, this study identified the Lm. reuteri strain WLR01 as having the potential to alleviate anxiety-like behavior and intestinal symptoms through the analysis of Lm. reuteri genotypes and phenotypes, as well as validation in mouse models, thereby laying the foundation for future clinical applications.
Keywords: Limosilactobacillus reuteri; anxiety-like behavior; gastrointestinal symptoms; gut microbiota; probiotics; strain-specific.
Conflict of interest statement
Yan-Yi Zheng, Si-Lu Zhang, and Guo-Xun Xiao are staff of WONDERLAB Innovation Center for Healthcare, an organization that is dedicated to innovation and commercialization of probiotics and nutrition supplements and partly supported this research. The funders had no role in the decision on publication of the study.
Figures



References
-
- Ferrari S., Mule S., Parini F., Galla R., Ruga S., Rosso G., Brovero A., Molinari C., Uberti F. The influence of the gut-brain axis on anxiety and depression: A review of the literature on the use of probiotics. J. Tradit. Complement. Med. 2024;14:237–255. doi: 10.1016/j.jtcme.2024.03.011. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

